植物修复技术因其修复面积广、操作简便、对环境干扰小、原位修复等优点而备受青睐。目前该技术还处于试验阶段,在国家“863”“973”计划支持下,植物修复研发工作不断推进,产官学研各类机构给予重视,取得了瞩目的成果。根据超富集植物数据库(www.hyperaccumulators.org)统计,全球已发现700多种超富集植物[1]。植物修复在高效提取土壤中重金属的同时产生了含高浓度重金属的生物质,处置污染土壤修复后含重金属的植物(简称修复植物)成为一个新的问题[2]。传统处置方法,如热解法、气化法、焚烧法,基本上是从减量化、无害化、部分资源化角度出发,而资源化处置技术,如热液改质法、植物冶金等,更多从资源化利用、实现经济价值的角度出发。本文对几种常用的修复植物处置方法进行概述,如堆肥法、压缩填埋法、焚烧法、气化法、热解法、植物冶金、热液改质法以及其他资源化处置方法,并指出各种方法的优缺点以及目前研究所存在的问题,并对未来研究发展进行展望。
1 传统处置方法 1.1 堆肥法堆肥法是一种高效且环境友好的固体废物处置方法,该方法利用微生物将固体废弃物中的有机物稳定化、腐殖化并产生有机肥料,可实现对修复植物的减容与减量[3]。
Cao等[4]利用堆肥法处理含As蜈蚣草后,发现堆肥后残渣中总As和水溶性As的含量分别降低了25% 和32%,绝大部分损失的As都转移到了渗滤液中,需通过高成本技术进行后续处理,防止二次污染。因此,有学者为改进修复植物堆肥法,减少堆肥后重金属带来的危害,发现添加石灰、生物质焚烧后飞灰、生物炭、赤泥等物质进行混合堆肥,可增加残渣中重金属残渣态含量,从而减少水溶态重金属的浸出,降低了环境风险与后续处理成本[5-7]。Wei等[8]研究不同堆肥源腐殖质和堆肥产生的耐重金属菌对重金属离子(Cu2+、Zn2+、Pb2+等)的吸收作用,发现重金属耐性菌较腐殖质与重金属有更好的结合能力,而腐殖质作为活化剂可以提高耐重金属菌的多样性和生物量,进而促进重金属离子被吸附,两者协同作用可减少60%~ 80% 的重金属浸出。Yang等[9]用类芽孢杆菌属结合床料(海绵、沸石、棉花)减少了渗滤液中重金属的含量,其中海绵和棉花与类芽孢杆菌属的结合减少了19.1%~26.4% Cr的浸出,这些研究为修复植物的堆肥处置提供了新思路。
堆肥法处置修复植物具有减容减量、占地面积小等优点。但其处理周期过长(通常需要2~3个月)、反应设备昂贵,堆肥残渣仍含有高浓度的重金属,需后续再处理,且迄今国家尚未颁布修复植物堆肥农用的相关标准,这些缺点限制了堆肥法处置修复植物的应用[10]。
1.2 压缩填埋压缩填埋法简单易行,是最常见的固体废物处置方法,常用于城市生活垃圾的处置。利用该法处置修复植物最早由Salt等[11]于1995年提出,但该方法运输成本较高,压缩所产生的渗滤液可能会在运输过程中泄露造成二次污染,所以很少应用于处理修复植物。
压缩填埋系统主要分为压缩储存系统和渗滤液收集系统,在压缩修复植物时会产生高浓度重金属与螯合剂化合的复合物。有研究表明,在压缩超富集植物所产生的渗滤液中,Cd、Pb、Ni等金属都主要以可溶态或生物可利用态的形式存在[12-14],需对其进行再次处理,防止二次环境污染。迄今,直接压缩填埋处置修复植物的可行性仍有待商榷。
与堆肥法相比,两者优点相似且压缩填埋的处置时间更短,但其产生的含重金属的渗滤液存在更高的二次污染风险,且未实现循环再利用。对于压缩后的修复植物,其重金属含量是否满足安全填埋标准也是一个新的问题。
1.3 焚烧法焚烧法是处置固体废物最便捷的方法,是一种无害化、减量化的热处理技术,且处理过程中可产生热能并回收利用(图 1)[15]。相较于堆肥和压缩填埋技术,焚烧法的处置效率高,减量化可达99%,便于运输与贮存[16]。但由于焚烧修复植物时会排放含重金属的飞灰、CO、NOx等污染物,造成二次环境污染,留在灰分中的金属化合物也具有易浸出特性,限制了灰分的再利用。

|
图 1 焚烧法、气化法、热解法反应过程 Figure 1 Reaction processes of incineration, gasification and pyrolysis |
近年来,不少学者研究了修复植物在焚烧过程中温度、生物质组成、添加剂等因素对重金属迁移转化的影响。Zhong等[17]焚烧超富集植物伴矿景天(Sedum plumbizincicola)发现,随着焚烧温度的增加,Zn、Cd、Pb的挥发程度升高,留在灰中的重金属多数(约99%)以硫化物、金属单质及氧化物的形式存在。灰分的形成与无机元素和碱金属元素相关,如Cl、K、Na、P、S等[18]。Hu等[19]的研究指出,氯含量是重金属以氯化物的形式挥发到大气中的关键因素。Luan等[20]研究了P、S、Cl在焚烧过程中对重金属的迁移转化影响,发现重金属的挥发对Cl有很强的依赖性,尤其是Cd、Pb、Ni;S在一定程度上也有利于重金属的挥发,但过量的氧抑制S对凝聚态重金属释放的促进作用;相反,由于矿物的形成,P对重金属有重要的固化作用。与通过减少修复植物中碱金属和无机元素的含量相比,添加固定剂辅助焚烧降低重金属的挥发更便捷有效。Zhu等[21]将γ-Al2O3混合含Cd黑麦草进行辅助焚烧,发现Cd在900 ℃下的回收率从低于10%提升至约40%,其中80%以上的Cd皆以残渣态的稳定形式存于底灰中。Jagodzińska等[22]的焚烧实验发现,硫酸铵对Hg、Cu和Cr具有固定能力,高岭土对Cd、Co和V具有固定能力,高岭石对Pb具有固定能力。
虽然通过添加固定剂辅助焚烧的方式能减少重金属的挥发,但其固定机理与形态结构尚不明确,且灰中易浸出部分金属,依然存在超标风险。Ma等[23]研究已尝试对灰分中的重金属进行固化稳定处理,避免二次环境污染。在实际工程应用中,焚烧产生的飞灰易堵塞腐蚀烟气收集系统,底灰易结垢残留在炉底引起腐蚀,这些是焚烧法目前处置修复植物中待解决的问题。
1.4 气化法在高温(>700 ℃)缺氧与气化剂的作用下对生物质进行部分氧化称为气化反应。气化法是介于焚烧法和高温热解法之间的一种热处理方法,能有效地对生物质进行减容减量并产生具有经济价值的高浓度可燃气体(H2、CO、CH4等),是生物质向可燃气体转化的重要途径(图 1)[24]。
目前常用的气化技术有固定床、流化床、气流床气化技术,不同气化技术对气化产物具有重要的影响[25]。Cui等[26]用固定床气化含重金属Cd、Zn的东南景天(Sedum alfredii),发现在700 ℃时,残渣中的Cd不足总量的2.0%;而在600 ℃时,95%以上的Zn以稳定态保留在残渣中,且Zn浸出浓度只有0.1~18.8 mg· L-1,低于危险废物填埋污染控制标准(GB 18598— 2001)限值。Lin等[27]用流化床气化技术研究了硅砂、沸石、氧化钙、煅烧煤和活性炭五种床料在气化过程中对重金属的挥发与产出氢气的影响,发现活性炭是捕捉重金属的最佳床料,产出的氢气占比达53.1%。Jiang等[28]用气流床技术气化多种修复植物,研究重金属的固气相变温度,发现当气化温度小于1 000 ℃时,Cd、Pb、Zn和As均易挥发,而Mn、Cu、Co和Ni均不易挥发。重金属及其化合物的挥发不仅受反应温度的影响,也受反应气流速度、床料类型和粒径尺寸、反应压力等的影响[28-31]。此外,一些新气化工艺技术逐渐出现,如Sun等[32]首次提出了新型自热CaO环生物质气化技术,以CO2为气化剂,利用CaO与气体中高浓度CO2反应释放热量为生物质气化提供能量,使合成气产出从0.21 kg·h-1增加到0.90 kg·h-1。Zhang等[33]用超临界水气化蓝藻,在500 ℃、23 MPa、持续10 min的条件下获得了2.92 mol·kg-1 H2,占总气量的33.3%。Zhang等[34]用微波辅助化学循环气化法,以赤铁矿为载氧剂,促进了H2和CO的生成,并发现挥发到生物气中90%的重金属可以用活性炭去除。
因此,不同工艺对气化产物和转化效率均有显著影响,其中修复植物原料、气化参数(温度、气化剂等)是重要影响因素。修复植物气化技术有较好的应用前景,关键在于根据植物特性和不同重金属迁移转化特征来确定适宜的工艺参数,并解决工艺中产生的有毒有害气体(如芳烃、气态金属化合物)、焦油等堵塞气流管线引起的腐蚀问题[35-36]。
1.5 热解法热解法,亦称高温分解法,是在高温厌氧条件下对生物质进行高温降解的一种热处理技术,实质是生物质的炭化。如图 1所示,相比于焚烧和气化这两种热处理技术,热解反应是在密闭环境中进行,不会向环境排放有毒有害气体,产出的生物油和生物气经处理后可作为生物能源使用,含重金属的生物炭可资源化利用,因而热解法是一种高效、绿色且资源友好的修复植物处置方法。
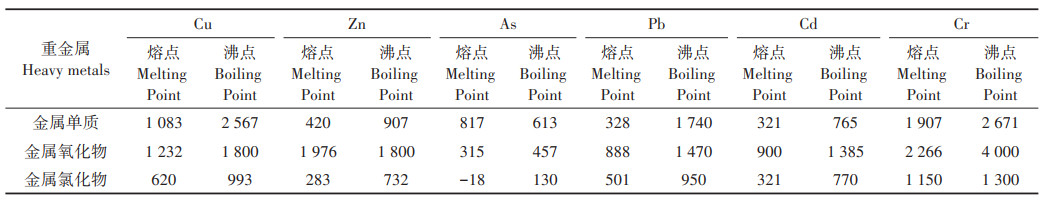
不同热解反应器和工艺会对热解产物品质产生不同的影响,目前常用的热解工艺主要分为慢速热解、快速热解和闪速热解,热解反应器主要是固定床反应器(例如管式炉),关于螺旋反应器和连续反应器的研究报道较少[37-38]。Kuppens等[39]的研究表明,固定床快速热解可有效地防止重金属的挥发,从而将重金属更多地保留在生物炭中。Wang等[40]在600 ℃条件下,用管式炉慢速热解Mn超富集植物垂序商陆(Phytolacca acinosa Roxb.),所得到的生物炭通过TCLP实验发现可浸出的Mn不到总量的0.15%,而其他不易挥发的金属,90% 以上都稳定赋存于生物炭中。不同的重金属及其化合物有不同的熔沸点(表 1),综合诸多学者的研究成果,常见几种重金属和As元素挥发性强弱排序依次为Cd>As>Pb>Zn>Cr>Cu> Ni[41]。生物质中非金属元素(如S、Cl、P)含量是影响重金属挥发的关键因素,Cl促进重金属挥发,而P对重金属表现为固定作用。所以,磷酸盐、碱性氧化物等作为添加剂可以与Pb、Cd、As元素在辅助热解过程中形成稳定的结晶化合物而保留在生物炭中,以便于后续的资源化利用以及安全处置[42-44]。故有学者将固定床慢速热解后含重金属的生物炭用作催化剂、活性炭、吸附剂或直接无害化填埋[40, 45-46]。另外,一些新兴的技术也逐渐出现在最近的研究中,Zeng等[47]用太阳能闪速热解(50 ℃·s-1)柳树研究重金属对生物气产量的影响,结果表明,在1 200 ℃、持续5 min的条件下,Cu和Ni的存在分别使得H2和CO的总产量增加了14.8%和34.5%,达10.3 mol·kg-1和12.2 mol·kg-1。
|
|
表 1 部分元素的氧化物、氯化物熔沸点(℃) Table 1 Transition point of partial elements′ oxides and chlorides(℃) |
因此,在不同的热解工艺条件下,可以实现不同目标产物的产出,但影响重金属在热解过程中的迁移转化以及热解产物品质的主要原因在于热解温度和重金属本身的理化性质,热解时间、升温速率、反应气氛和添加剂以及原料性质等也是影响产物品质的原因[41]。热解后留在生物炭中的重金属大部分都以稳定态的形式存在,其环境风险评价指数(RAC)、潜在生态风险指数(RI)等指标[26, 48-49]尚可接受。存于生物炭中的重金属还可以通过酸式(pH=1)浸提后再调整pH到11,沉淀分离重金属[50]。少量存于生物油中的重金属可通过阳离子交换、吸附、溶剂萃取和化学沉淀技术分离,以得到更加清洁的生物油能源[51-52]。
热解法能显著减少修复植物的生物量并可进行后续资源化利用,获得附加值[53]。但该技术也存在一些不足,如生物质的含水率需控制在10% 以下、热解产生的焦油会堵塞管道、运行设备昂贵等,且目前热解技术的研究还处于实验室阶段,缺乏实际工程应用。
2 资源化处置技术 2.1 植物冶金植物冶金是利用修复植物进行浸出回收目标重金属的一种修复植物处置方法[54]。从1983年Chaney[55]提出植物冶金以来,其理论研究和实践都有了较大的提升。目前,全世界共发现700多种修复植物,其中60% 以上都为Ni的超富集植物。据统计,因采矿区域、种植方式、冶炼方法的不同,植物采镍的利润在984~1 806美元·hm-2之间[56-57]。为弥补我国镍矿资源的不足,目前有关植物冶金的研究大部分为Ni,极少部分为Au、Cu、Ag[58-59]。
Martijn等[60]利用水热法成功地回收了镍并获取了生物质燃料,但此方法对设备和控制条件要求高,运行成本高,技术也尚未成熟。Barbaroux等[61]直接用0.5 mol·L-1硫酸浸提修复植物中的Ni,然后再通过电化学沉淀法从中回收Ni,但是由于回收效率低未被广泛使用。Ni在热处理过程中不易挥发,焚烧含Ni修复植物后其灰分中Ni的质量分数是植物体内的10~20倍,因此很多学者支持从焚烧灰分中回收Ni。此外,Hazotte等[62]提出了一种新颖的湿法冶金工艺,通过胶结和沉淀从修复植物中成功地回收了Zn、Cd,这为今后修复植物的回收利用提供了新思路。
植物冶金相较于直接从矿场采矿冶金,对环境的干扰和破坏程度低且操作简便省力,但超富集植物的选取对植物冶金至关重要,需具备超强的富集能力和较大的生物量。随着冶金技术的进步,针对不同目标金属的提取技术也在不断发展。在实际应用中,还需对植物冶金技术工艺参数进行优化,降低冶金成本,使植物冶金技术大规模商业化应用,达到最优的环境经济效益。
2.2 热液改质技术热液改质是指在高压反应釜中利用超临界或亚临界状态水将生物质转变为高热值燃料的一种技术[63]。在热液改质法中,重金属的去向主要是生物炭和生物油,温度、水料比、压力、持续时间、K2CO3浓度是影响生物油质量和水热炭理化性质的重要因素[64]。
随着热液改质技术的发展,越来越多的学者利用此技术研究修复植物的处置。Qian等[65]利用热液改质技术处置修复植物伴矿景天(Sedum plumbizincicola),发现在200 ℃时,约90% 的Zn转移到了生物油中,且得到比表面积达930 m2 · g-1、捕捉CO2达3 mmol·g-1的生物炭。Su等[66]在440 ℃、25 MPa条件下热液改质修复植物伴矿景天(Sedum plumbizincicola),产出了2.74 mol·kg-1的H2,且99.2% 以上的重金属都保留在水热炭中。Chen等[67]用热液改质技术将修复植物(含Cd稻草和含Cu海州香薷Elsholtzia splendens)中100% Cd和95% Cu固定在生物炭渣中,并验证了重金属以单质的形式存于炭渣中,可作金属负载炭催化剂,同时得到热值为38.1 MJ·kg-1的生物油。Chen等[68]的研究发现热液改质产出的生物油成分多为石油原油成分,有望成为化石燃料的替代品。同时,Pb、Ni等重金属通过促进加氢反应增加有机酸的生成,可提升生物油的品质[69]。为获得更加清洁的生物油,提高热液化效率,阴阳离子交换、吸附、溶剂萃取和化学沉淀等方法可以分离生物油中的重金属[69-71]。
与热解法相比,由于反应温度较低,重金属挥发量较少,且无需脱水预处理,热液改质法处理修复植物具有独特的优势。然而,热液改质法也存在一些影响其应用的不足之处。该方法通常需要高温高压的反应条件,运行成本高。反应过程中生成的盐在超临界水中溶解度较低,极易发生沉淀,当这些沉淀物与焦炭结合时,会阻塞反应器,增加设备运行成本。另外,生物质中的S和Cl也会在反应中转化为HCl和H2SO4,对设备造成腐蚀。
2.3 其他资源化处置技术将修复植物制成合成金属纳米材料是近年来新开发的绿色资源化处置技术,其成本和对环境的危害比传统的金属纳米材料技术要低[72]。Qu等[73]用含Zn和Cu的修复植物制备出高纯的粒径为110 nm负载Cu和ZnO的合成金属碳纳米管材料。Chen等[74]先后通过热解与水热硫化过程将Cd超富集植物伴矿景天制成具有六方立体结构的CdS@C纳米复合材料,该材料有较好的光催化能力,可降解颜料废水。修复植物制备合成金属纳米材料实现了减量化、无害化与资源化,甚至变废为宝,这些研究打破了传统的资源回收方式与思维方法,打开了修复植物处置方法的新思路,推进了植物修复技术的潜在应用。另外,Losfeld等[75]从Ni超富集植物中制备路易斯酸催化剂,该催化剂可用于有机化学合成,具有较好的经济效益和生态效益,但该催化剂的稳定性、活性、反应机理等性质还需深入研究。还有学者正致力于常压下微波、超声、直接水热法等处理Ni超富集植物以获得不同的Ni产品[76]。这些研究和探索为修复植物资源化处置拓宽了思路,有望通过未来深入研究而进一步走向实际应用。
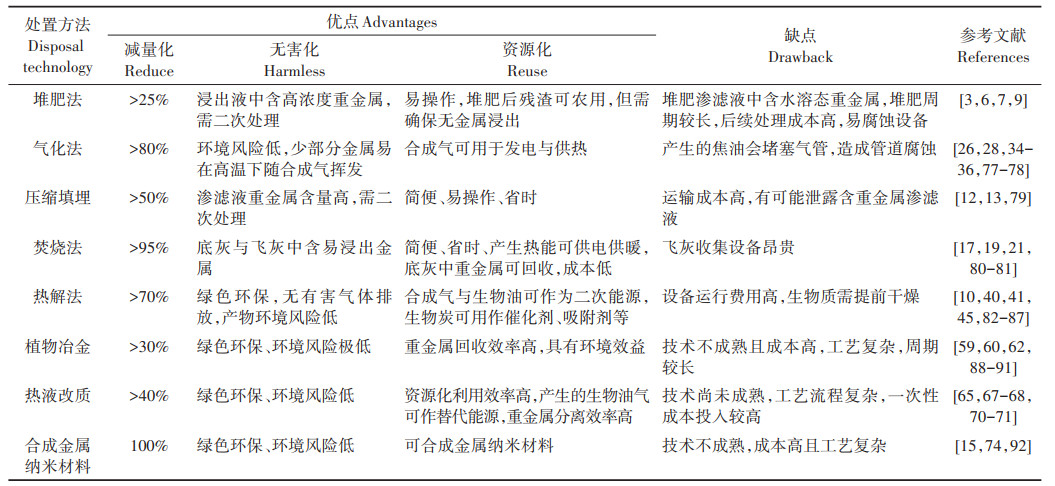
3 处置技术方法对比基于上述分析,表 2归纳和对比了几种主要修复植物处置技术的特点,以期为今后的处置技术选择提供一定的参考。
|
|
表 2 不同修复植物处置技术比较 Table 2 Comparison of disposal technologies of polluted plants after phytoextraction |
植物修复因其简便易操作且可原位修复的特点而备受青睐,但修复后产生含大量重金属的植物会带来新的环境风险。在修复植物处置技术中,目前的研究主要集中在热处理技术。通常,热处理可以增强修复植物中重金属的稳定性,从而降低其在固体产品中的生物可利用性。热处理后的固相产物(即生物炭和水热炭)可作为吸附剂和催化剂,且产生的生物油和生物气具有合成各种生物燃料和化学品的良好潜力。重金属本身性质及修复植物体内重金属含量的高低也是选择处置技术时需考虑的重要因素。结合修复植物处置技术发展现状,本文对其中应用前景较好的热解法与热液改质法进行了展望,并提出以下建议:
(1)对于热解法,应深入研究优化热解工艺参数,以减少重金属释放到液相与气相中,降低对设备的腐蚀,提高热解效率。同时,添加物辅助热解的固定机理还需系统化研究,明确常见重金属与添加剂的相互固定关系,为今后将修复植物制成合成材料等奠定基础,实现“变废为宝”。
(2)热液改质法具有较好的经济效益,所得到的三相产物均有较高的附加值。今后应着重研究热液反应后各相产品的回收技术以及设备的革新,拓展工业化研究,优化热液技术工艺参数与工艺流程,减弱产物对设备的腐蚀性,降低运行与一次性投入成本。
| [1] |
Reeves R D, Baker A J M, Jaffre T, et al. A global database for plants that hyperaccumulate metal and metalloid trace elements[J]. New Phytologist, 2017, 218: 407-411. |
| [2] |
Huang R, Dong M l, Mao P, et al. Evaluation of phytoremediation potential of five Cd(hyper) accumulators in two Cd contaminated soils[J]. Science of the Total Environment, 2020, 721: 137581. DOI:10.1016/j.scitotenv.2020.137581 |
| [3] |
Chen X, Zhao Y, Zeng C, et al. Assessment contributions of physicochemical properties and bacterial community to mitigate the bioavailability of heavy metals during composting based on structural equation models[J]. Bioresource Technology, 2019, 289: 121657. DOI:10.1016/j.biortech.2019.121657 |
| [4] |
Cao X, Shiralipour A, Harris W. Biomass reduction and arsenic transformation during composting of arsenic-rich hyperaccumulator Pteris vittata L[J]. Environmental Science and Pollution Research, 2009, 17(3): 586-594. |
| [5] |
Singh J, Kalamdhad A S. Effect of lime on speciation of heavy metals during composting of water hyacinth[J]. Frontiers of Environmental Science & Engineering, 2016, 10(1): 93-102. |
| [6] |
Soares M A, Quina M J, Quinta-Ferreira R M. Immobilisation of lead and zinc in contaminated soil using compost derived from industrial eggshell[J]. Journal of Environmental Management, 2015, 164: 137-145. |
| [7] |
Asquer C, Cappai G, Carucci A, et al. Biomass ash characterisation for reuse as additive in composting process[J]. Biomass and Bioenergy, 2019, 123: 186-194. DOI:10.1016/j.biombioe.2019.03.001 |
| [8] |
Wei Y, Zhao Y, Zhao X, et al. Roles of different humin and heavy-metal resistant bacteria from composting on heavy metal removal[J]. Bioresource Technology, 2020, 296: 122375. DOI:10.1016/j.biortech.2019.122375 |
| [9] |
Yang K, Zhu L, Zhao Y, et al. A novel method for removing heavy metals from composting system: The combination of functional bacteria and adsorbent materials[J]. Bioresource Technology, 2019, 293: 122095. DOI:10.1016/j.biortech.2019.122095 |
| [10] |
Sas-Nowosielska A, Kucharski R, Malkowski E, et al. Phytoextraction crop disposal: An unsolved problem[J]. Environmental Pollution, 2004, 128(3): 373-379. DOI:10.1016/j.envpol.2003.09.012 |
| [11] |
Salt D E, Blaylock M, Kumar N P B A, et al. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants[J]. Biotechnology, 1995, 13(5): 468-474. |
| [12] |
Geraldine S J V, Alain M, Martine M, et al. Accumulation forms of Zn and Pb in Phaseolus vulgaris in the presence and absence of EDTA[J]. Environmental Science and Technology, 2001, 35(13): 2854-2859. DOI:10.1021/es000219d |
| [13] |
Zhao F J, Lombi E, Breedon T, et al. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri[J]. Plant, Cell and Environment, 2000, 23: 507-514. DOI:10.1046/j.1365-3040.2000.00569.x |
| [14] |
Raskin I, Smith R D, Salt D E. Phytoremediation of metals: Using plants to remove pollutants from the environment[J]. Current Opinion in Biotechnology, 1997, 8(2): 221-226. DOI:10.1016/S0958-1669(97)80106-1 |
| [15] |
李宁, 吴龙华, 孙小峰, 等. 修复植物产后处置技术现状与展望[J]. 土壤, 2005, 37(6): 687-592. LI Ning, WU Long-hua, SUN Xiaofeng, et al. Techniques for disposal or reuse of phytoremediating plants: Present and Future[J]. Soils, 2005, 37(6): 687-592. |
| [16] |
Lei M, Dong Z P, Jiang Y, et al. Reaction mechanism of arsenic capture by a calcium-based sorbent during the combustion of arseniccontaminated biomass: A pilot-scale experience[J]. Frontiers of Environmental Science and Engeering, 2019, 13(2): 24. DOI:10.1007/s11783-019-1110-y |
| [17] |
Zhong D X, Zhong Z P, Wu L H, et al. Thermal characteristics of hyperaccumulator and fate of heavy metals during thermal treatment of Sedum plumbizincicola[J]. International Journal of Phytoremediation, 2015, 17(8): 766-776. DOI:10.1080/15226514.2014.987373 |
| [18] |
Vassilev S V, Vassileva C G, Song Y C, et al. Ash contents and ashforming elements of biomass and their significance for solid biofuel combustion[J]. Fuel, 2017, 208: 377-409. DOI:10.1016/j.fuel.2017.07.036 |
| [19] |
Hu Y, Wang J, Deng K, et al. Characterization on heavy metals transferring into flue gas during sewage sludge combustion[J]. Energy Procedia, 2014, 61: 2867-2870. DOI:10.1016/j.egypro.2014.12.325 |
| [20] |
Luan J, Li R, Zhang Z, et al. Influence of chlorine, sulfur and phosphorus on the volatilization behavior of heavy metals during sewage sludge thermal treatment[J]. Waste Management and Research, 2013, 31(10): 1012-1018. DOI:10.1177/0734242X13493955 |
| [21] |
Zhu Z C, Huang Y J, Zha J R, et al. Emission and retention of cadmium during the combustion of contaminated biomass with mineral additives[J]. Energy & Fuels, 2019, 33(12): 12508-12517. |
| [22] |
Jagodzińska K, Mroczek K, Nowińska K, et al. The impact of additives on the retention of heavy metals in the bottom ash during RDF incineration[J]. Energy, 2019, 183: 854-868. DOI:10.1016/j.energy.2019.06.162 |
| [23] |
Ma W, Shi W, Shi Y, et al. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash[J]. Journal of Hazardous Materials, 2020, 408: 124809. |
| [24] |
Sansaniwal S K, Rosen M A, Tyagi S K. Global challenges in the sustainable development of biomass gasification: An overview[J]. Renewable and Sustainable Energy Reviews, 2017, 80: 23-43. DOI:10.1016/j.rser.2017.05.215 |
| [25] |
Situmorang Y A, Zhao Z, Yoshida A, et al. Small-scale biomass gasification systems for power generation[J]. Renewable and Sustainable Energy Reviews, 2020, 117: 109486. DOI:10.1016/j.rser.2019.109486 |
| [26] |
Cui X Q, Shen Y, Yang Q Y, et al. Simultaneous syngas and biochar production during heavy metal separation from Cd/Zn hyperaccumulator(Sedum alfredii)by gasification[J]. Chemical Engineering Journal, 2018, 347: 543-551. DOI:10.1016/j.cej.2018.04.133 |
| [27] |
Lin C L, Wu M H, Weng W C. Effect of the type of bed material in two-stage fluidized bed gasification reactors on hydrogen gas synthesis and heavy metal distribution[J]. Interantional Journal of Hydrogen Energy, 2019, 44(11): 5633-5639. DOI:10.1016/j.ijhydene.2018.07.199 |
| [28] |
Jiang Y, Ameh A, Lei M, et al. Solid-gaseous phase transformation of elemental contaminants during the gasification of biomass[J]. Science of the Total Environment, 2016, 563/564: 724-730. DOI:10.1016/j.scitotenv.2015.11.017 |
| [29] |
Wu M H, Lin C L, Zeng W Y. Effect of waste incineration and gasification processes on heavy metal distribution[J]. Fuel Process Technology, 2014, 125: 67-72. DOI:10.1016/j.fuproc.2014.03.027 |
| [30] |
Froment K, Defoort F, Bertrand C, et al. Thermodynamic equilibrium calculations of the volatilization and condensation of inorganics during wood gasification[J]. Fuel, 2013, 107: 269-281. DOI:10.1016/j.fuel.2012.11.082 |
| [31] |
Liu S, Wang Y, Yu L, et al. Volatilization of mercury, arsenic and selenium during underground coal gasification[J]. Fuel, 2006, 85(10/11): 1550-1558. |
| [32] |
Sun H M, Wu C F. Correction to autothermal CaO looping biomass gasification for renewable syngas production[J]. Environmental Science and Technology, 2020, 54(7): 4695. DOI:10.1021/acs.est.9b06120 |
| [33] |
Zhang H W, Zhang X M, Ding L, et al. Characteristics of cyanobacterial biomass gasification in sub-and supercritical water[J]. Energy & Fuels, 2019, 33(4): 3239-3247. |
| [34] |
Zhang B, Zhang J, Zhong Z, et al. Syngas production and trace element emissions from microwave-assisted chemical looping gasification of heavy metal hyperaccumulators[J]. Science of the Total Environment, 2019, 659: 612-620. DOI:10.1016/j.scitotenv.2018.12.176 |
| [35] |
Mahesh K S, Madhu G M, Roy S. Fouling behaviour, regeneration options and on-line control of biomass-based power plant effluents using microporous ceramic membranes[J]. Seperation and Purification Technology, 2007, 57(1): 25-36. DOI:10.1016/j.seppur.2007.03.002 |
| [36] |
Baumnhakl K S. Tar analysis from biomass gasification by means of online fluorescence spectroscopy[J]. Optics and Lasers in Engineering, 2011, 49(7): 885-891. DOI:10.1016/j.optlaseng.2011.02.015 |
| [37] |
Liu Z, Wang L A, Xiao H, et al. A review on control factors of pyrolysis technology for plants containing heavy metals[J]. Ecotoxicology and Environmental Safety, 2020, 191: 110181. DOI:10.1016/j.ecoenv.2020.110181 |
| [38] |
Xin X, Dell K, Udugama I A, et al. Transforming biomass pyrolysis technologies to produce liquid smoke food flavouring[J/OL]. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2020.125368.
|
| [39] |
Kuppens T, Van Dael M, Vanreppelen K, et al. Techno-economic assessment of fast pyrolysis for the valorization of short rotation coppice cultivated for phytoextraction[J]. Journal of Cleaner Production, 2015, 88: 336-344. DOI:10.1016/j.jclepro.2014.07.023 |
| [40] |
Wang S S, Gao B, Li Y C, et al. Biochar provides a safe and valueadded solution for hyperaccumulating plant disposal: A case study of Phytolacca acinosa Roxb.(Phytolaccaceae)[J]. Chemosphere, 2017, 178: 59-64. DOI:10.1016/j.chemosphere.2017.02.121 |
| [41] |
尚鹏鹏, 盛奎川, 刘蒋龙, 等. 修复植物热解过程中重金属迁移转化特性的研究进展[J]. 可再生能源, 2020, 38(3): 285-291. SHANG Peng-peng, SHENG Kui-chuan, LIU Jiang-long, et al. Research progress of heavy metal transformation and migration behavior during pyrolysis of phytoremediation plants[J]. Renewable Energy Resources, 2020, 38(3): 285-291. DOI:10.3969/j.issn.1671-5292.2020.03.001 |
| [42] |
Liu Y N, Guo Z H, Sun Y, et al. Stabilization of heavy metals in biochar pyrolyzed from phytoremediated giant reed(Arundo donax)biomass[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(3): 656-665. DOI:10.1016/S1003-6326(17)60073-6 |
| [43] |
Shi L N, Wang L J, Zhang T, et al. Reducing the bioavailability and leaching potential of lead in contaminated water hyacinth biomass by phosphate-assisted pyrolysis[J]. Bioresource Technology, 2017, 241: 908-914. DOI:10.1016/j.biortech.2017.06.025 |
| [44] |
Zhao L, Cao X D, Zheng W, et al. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil[J]. ACS Sustainabale Chemistry and Engineering, 2016, 4(3): 1630-1636. DOI:10.1021/acssuschemeng.5b01570 |
| [45] |
Gong X M, Huang D L, Liu Y G, et al. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption[J]. Bioresource Technology, 2018, 253: 64-71. DOI:10.1016/j.biortech.2018.01.018 |
| [46] |
Xing R Z, Li J X, Yang X G, et al. Preparation of high-performance CdS@C catalyst using Cd-enriched biochar recycled from plating wastewater[J]. Frontiers in Chemistry, 2020, 8: 140. DOI:10.3389/fchem.2020.00140 |
| [47] |
Zeng K, Li R, Minh D P, et al. Solar pyrolysis of heavy metal contaminated biomass for gas fuel production[J]. Energy, 2019, 187: 116016. DOI:10.1016/j.energy.2019.116016 |
| [48] |
He J, Strezov V, Kan T, et al. Slow pyrolysis of metal(loid)-rich biomass from phytoextraction: Characterisation of biomass, biochar and bio-oil[J]. Energy Procedia, 2019, 160: 178-185. DOI:10.1016/j.egypro.2019.02.134 |
| [49] |
Du J, Zhang L, Liu T, et al. Thermal conversion of a promising phytoremediation plant(Symphytum officinale L.)into biochar: Dynamic of potentially toxic elements and environmental acceptability assessment of the biochar[J]. Bioresource Technology, 2019, 274: 73-82. DOI:10.1016/j.biortech.2018.11.077 |
| [50] |
Yang Y, Ge Y C, Tu P F, et al. Phytoextraction of Cd from a contaminated soil by tobacco and safe use of its metal-enriched biomass[J]. Journal of Hazardous Materials, 2019, 363: 385-393. DOI:10.1016/j.jhazmat.2018.09.093 |
| [51] |
Kim J Y, Oh S, Park Y K. Overview of biochar production from preservative-treated wood with detailed analysis of biochar characteristics, heavy metals behaviors, and their ecotoxicity[J]. Journal of Hazardous Materials, 2020, 384: 121356. DOI:10.1016/j.jhazmat.2019.121356 |
| [52] |
Cui X Q, Zhang J W, Wang X T, et al. A review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products[J]. Journal of Hazardous Materials, 2020, 405: 123832. |
| [53] |
Lievens C, Yperman J, Cornelissen T, et al. Study of the potential valorisation of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: Part Ⅱ: Characterisation of the liquid and gaseous fraction as a function of the temperature[J]. Fuel, 2008, 87(10/11): 1906-1916. |
| [54] |
Brooks R R, Charmbers F M, Nicks J L, et al. Phytomining trends in plant science[J]. Trends in Plant Science, 1998, 3(9): 359-362. DOI:10.1016/S1360-1385(98)01283-7 |
| [55] |
Chaney R L. Plant uptake of inorganic waste constituents[J]. Journal of Environmental Protection, 1983, 7(5): 50-76. |
| [56] |
Bani A, Echevarria G. Can organic amendments replace chemical fertilizers in nickel agromining cropping systems in Albania?[J]. International Journal of Phytoremediation, 2019, 21(1): 43-51. DOI:10.1080/15226514.2018.1523871 |
| [57] |
Nkrumah P N, Baker A J M, Chaney R L, et al. Current status and challenges in developing nickel phytomining: An agronomic perspective[J]. Plant Soil, 2016, 406(1): 55-69. |
| [58] |
Rosenkranz T, Hipfinger C, Ridard C, et al. A nickel phytomining field trial using Odontarrhena chalcidica and Noccaea goesingensis on an Austrian serpentine soil[J]. Journal of Environmental Management, 2019, 242: 522-528. DOI:10.1016/j.jenvman.2019.04.073 |
| [59] |
González V E, Alarcón A, Ferrera C R, et al. Induced accumulation of Au, Ag and Cu in Brassica napus grown in a mine tailings with the inoculation of Aspergillus niger and the application of two chemical compounds[J]. Ecotoxicology & Environmental Safety, 2018, 154: 180. |
| [60] |
Martijn L C, Kunio A. Hydrothermal processing of nickel containing biomining or bioremediation biomass[J]. Biomass and Bioenergy, 2001, 21(1): 73-80. DOI:10.1016/S0961-9534(01)00010-1 |
| [61] |
Barbaroux R, Mercier G, Blais J F, et al. A new method for obtaining nickel metal from the hyperaccumulator plant Alyssum murale[J]. Seperation and Purification Technology, 2011, 83: 57-65. DOI:10.1016/j.seppur.2011.09.009 |
| [62] |
Hazotte C, Laubie B, Rees F, et al. A novel process to recover cadmium and zinc from the hyperaccumulator plant Noccaea caerulescens[J]. Hydrometallurgy, 2017, 174: 56-65. DOI:10.1016/j.hydromet.2017.09.012 |
| [63] |
Zbigniew S, Gaëlle B A, Van E A, et al. Hydrothermal upgrading of biomass to biofuel: Studies on some monosaccharide model compounds[J]. Carbohydrate Research, 2004, 339(10): 1717-1726. DOI:10.1016/j.carres.2004.04.018 |
| [64] |
Cui X, Lu M, Khan M B, et al. Hydrothermal carbonization of different wetland biomass wastes: Phosphorus reclamation and hydrochar production[J]. Waste Management, 2020, 102: 106-113. DOI:10.1016/j.wasman.2019.10.034 |
| [65] |
Qian F, Zhu X, Liu Y, et al. Influences of temperature and metal on subcritical hydrothermal liquefaction of hyperaccumulator: Implications for the recycling of hazardous hyperaccumulators[J]. Environmental Science and Technology, 2018, 52(4): 2225-2234. DOI:10.1021/acs.est.7b03756 |
| [66] |
Su W, Liu P, Cai C, et al. Hydrogen production and heavy metal immobilization using hyperaccumulators in supercritical water gasification[J]. Journal of Hazardous Materials, 2021, 402: 123541. DOI:10.1016/j.jhazmat.2020.123541 |
| [67] |
Chen H, Wang X L, Lu X L, et al. Hydrothermal conversion of Cd-enriched rice straw and Cu-enriched Elsholtzia splendens with the aims of harmless treatment and resource reuse[J]. Industrial & Engineering Chemistry Research, 2018, 57(46): 15683-15689. |
| [68] |
Chen J B, Li S M. Characterization of biofuel production from hydrothermal treatment of hyperaccumulator waste(Pteris vittata L.) in sub-and supercritical water[J]. RSC Advances, 2020, 10(4): 2160-2169. DOI:10.1039/C9RA09410E |
| [69] |
Wang Y, Deng W, Wang B, et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(Ⅱ)ions in water[J]. Nature Communication, 2013, 4: 2141. DOI:10.1038/ncomms3141 |
| [70] |
Yang J G, Li J Y, Yang J Y, et al. Hydrothermal processing of arsenic containing bioremediation biomass: Pteris vittata[J]. Journal of Environmental Chemical Engineering, 2014, 2(3): 1358-1364. DOI:10.1016/j.jece.2014.04.011 |
| [71] |
Djandja S O, Wang Z C, Chen L, et al. Progress in hydrothermal liquefaction of algal biomass and hydrothermal upgrading of the subsequent crude bio-oil: A mini review[J]. Energy & Fuels, 2020, 34(10): 01973. |
| [72] |
Ahmed S, An nu, Ikram S, et al. Biosynthesis of gold nanoparticles: A green approach[J]. Journal of Photochemistry and Photobiology B-Biology, 2016, 161: 141-153. DOI:10.1016/j.jphotobiol.2016.04.034 |
| [73] |
Qu J, Luo C Q, Cong Q, et al. Recycling of the hyperaccumulator Brassica juncea L.: Synthesis of carbon nanotube-Cu/ZnO nanocomposites[J]. Journal of Material Cycles and Waste Management, 2014, 16(1): 162-166. DOI:10.1007/s10163-013-0156-3 |
| [74] |
Chen Z, Xing R Z, Tang J H, et al. Upcycling of Cd hyperaccumulator biomass into a CdS@C nanocomposite with high photocatalytic performance[J]. ACS Sustainable Chemistry and Engineering, 2020, 8(3): 1388-1395. DOI:10.1021/acssuschemeng.9b05518 |
| [75] |
Losfeld G, Escande V, Blache P V, et al. Design and performance of supported Lewis acid catalysts derived from metal contaminated biomass for Friedel-Crafts alkylation and acylation[J]. Catalysis Today, 2012, 189(1): 111-116. DOI:10.1016/j.cattod.2012.02.044 |
| [76] |
张鑫, 吕香英, 刘文深, 等. 基于植物采矿的"绿色治镍"研究进展[J]. 中山大学学报, 2017, 56(5): 20-29. ZHANG Xin, LÜ Xiangying, LIU Wen-shen, et al. Research progress of green metallurgical nickel based on phytomining[J]. ACTA Scientiarum Naturalium Universitatis Sunyatseni, 2017, 56(5): 20-29. |
| [77] |
Vervaeke P, Tack F M G, Navez F, et al. Fate of heavy metals during fixed bed downdraft gasification of willow wood harvested from contaminated sites[J]. Biomass and Bioenergy, 2006, 30(1): 58-65. DOI:10.1016/j.biombioe.2005.07.001 |
| [78] |
Ge H, Guo W, Shen L, et al. Experimental investigation on biomass gasification using chemical looping in a batch reactor and a continuous dual reactor[J]. Chemistry Engineering Journal, 2016, 286: 689-700. DOI:10.1016/j.cej.2015.11.008 |
| [79] |
Raskin I, Ensley B, Wiley J, et al. Phytoremediation of toxic metals using plants to clean up the environment[J]. Plant Science, 2001, 160(1): 1073-1075. |
| [80] |
Bie R S, Chen P, Song X F, et al. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment[J]. Journal of the Energy Institute, 2016, 89(4): 704-712. DOI:10.1016/j.joei.2015.04.006 |
| [81] |
Lu S, Du Y, Zhong D, et al. Comparison of trace element emissions from thermal treatments of heavy metal hyperaccumulators[J]. Environmental Science and Technology, 2012, 46(9): 5025-5031. DOI:10.1021/es202616v |
| [82] |
Du J, Zhang L, Ali A, et al. Research on thermal disposal of phytoremediation plant waste: Stability of potentially toxic metals(PTMs) and oxidation resistance of biochars[J]. Process Safety and Environmental Protection, 2019, 125: 260-268. DOI:10.1016/j.psep.2019.03.035 |
| [83] |
Duan L B, Li X L, Jiang Y, et al. Arsenic transformation behaviour during thermal decomposition of P. vittata, an arsenic hyperaccumulator[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 584-591. DOI:10.1016/j.jaap.2017.01.013 |
| [84] |
He J, Strezov V, Kan T, et al. Effect of temperature on heavy metal (loid)deportment during pyrolysis of Avicennia marina biomass obtained from phytoremediation[J]. Bioresource Technology, 2019, 278: 214-222. DOI:10.1016/j.biortech.2019.01.101 |
| [85] |
He J, Strezov V, Kumar R, et al. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products[J]. Journal of Cleaner Production, 2019, 234: 1235-1245. DOI:10.1016/j.jclepro.2019.06.285 |
| [86] |
Huang H, Yao W, Li R, et al. Effect of pyrolysis temperature on chemical form, behavior and environmental risk of Zn, Pb and Cd in biochar produced from phytoremediation residue[J]. Bioresource Technology, 2018, 249: 487-493. DOI:10.1016/j.biortech.2017.10.020 |
| [87] |
Li S J, Zhang T, Li J F, et al. Stabilization of Pb(Ⅱ)accumulated in biomass through phosphate-pretreated pyrolysis at low temperatures[J]. Journal of Hazardous Materials, 2017, 324: 464-471. DOI:10.1016/j.jhazmat.2016.11.014 |
| [88] |
杨建广, 杨建英, 彭长宏, 等. 从超富集植物Berkheya coddii中回收镍[J]. 中国有色金属学报, 2009, 19(4): 754-759. YANG Jianguang, YANG Jian-ying, PENG Chang-hong, et al. Recovery nickel from Bekheya coddii biomass[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(4): 754-759. DOI:10.3321/j.issn:1004-0609.2009.04.026 |
| [89] |
Chaney R L, Angle J S, Broadhurst C L, et al. Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies[J]. Journal of Environmental Quality, 2007, 36(5): 1429-1443. DOI:10.2134/jeq2006.0514 |
| [90] |
Yang J G. Heavy metal removal and crude bio-oil upgrading from Sedum plumbizincicola harvest using hydrothermal upgrading process[J]. Bioresource Technology, 2010, 101(19): 7653-7657. DOI:10.1016/j.biortech.2010.04.095 |
| [91] |
Yang J G, Yang J Y, Peng C H, et al. Recovery of zinc from hyperaccumulator plants: Sedum plumbizincicola[J]. Environmental Technology, 2009, 30(7): 693-700. DOI:10.1080/09593330902894349 |
| [92] |
刘维涛, 倪均成, 周启星, 等. 重金属富集植物生物质的处置技术研究进展[J]. 农业环境科学学报, 2014, 33(1): 15-27. LIU Weitao, NI Jun-cheng, ZHOU Qi-xing, et al. Research progress of disposal technology for heavy metal-enriched plant biomass[J]. Journal of Agro-Environment Science, 2014, 33(1): 15-27. |
 2021, Vol. 38
2021, Vol. 38