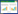文章信息
- 张宏祥, 李丽娟, 曾希柏, 白玲玉, 陕红, 王亚男, 吴翠霞, 苏世鸣
- ZHANG Hong-xiang, LI Li-juan, ZENG Xi-bai, BAI Ling-yu, SHAN Hong, WANG Ya-nan, WU Cui-xia, SU Shi-ming
- 土壤接种棘孢木霉菌降低小油菜砷胁迫及其可能机理
- Reliefs the Exposure Stress of Soils Arsenic on Brassica campestris L.Growth and Its Possible Mechanisms by Inoculation of Trichoderma asperellum SM-12F1
- 农业资源与环境学报, 2018, 35(2): 139-146
- Journal of Agricultural Resources and Environment, 2018, 35(2): 139-146
- http://dx.doi.org/10.13254/j.jare.2018.0007
-
文章历史
- 收稿日期: 2018-01-05
录用日期: 2017-02-08
砷是自然界中广泛存在的类金属元素,农田土壤中砷的过量累积会影响作物的正常生长,较高浓度的砷会影响种子萌发[1],破坏植物光合系统[2],降低农作物的产量和品质,严重时甚至导致植物死亡。土壤砷胁迫下,植株体内会产生过多的活性氧,后者具有极强的氧化性,可破坏蛋白质、DNA等生物大分子[3-4]。安堃达等[5]研究发现土壤添加50 mg·kg-1的外源砷可导致豇豆的生物量降低47%;郝玉波等[3]研究了砷对玉米生长及抗氧化体系的影响,结果表明,水培条件下高浓度砷(>4 mg·L-1)严重抑制玉米生长和其对P、K、Ca等元素的吸收。马茹茹等[6]研究发现,低浓度的外源砷促进小油菜生长,而高浓度的外源砷显著抑制小油菜生长。
随着农田土壤砷污染日趋严重,如何缓解砷对作物生长的毒害,减少砷在农作物中的累积已经引起了广泛关注。特别是针对某些对砷具有相对低吸收能力的作物而言,其对砷的吸收量并未超过国家相关标准,但生长明显受到土壤砷抑制,如何保障该类作物的健康生长,对于砷污染地区农田的安全生产与风险调控具有重要意义。张敏等[7]研究了外源硅缓解水稻幼苗砷毒性的效应,结果表明,外源添加硅可以促进水稻生长并降低了水稻对砷的吸收。硒可以显著提高作物抗氧化能力和抗逆性,减缓砷对作物的胁迫,这是因为部分抗氧化酶是以硒为酶辅因子,外源硒的加入促进了作物抗氧化酶的产生[8]。
此外,某些土壤微生物在调控土壤砷有效性、降低砷对作物生长胁迫方面亦表现出很大的潜力,并逐渐成为近年来研究的热点之一[9]。真菌因其生物量大、菌丝的成网结构、生命周期长等特点,在砷污染土壤修复中较其他类型微生物有着明显的优势[10]。某些真菌可以加速土壤磷的溶解、提高土壤酶活性、产生吲哚乙酸(IAA)等物质促进作物生长[11-12]。部分耐砷真菌可以降低土壤砷有效性并促进砷形态转化,从而降低砷的毒性[13]。但从总体来看,利用微生物的耐砷、促生特性来减缓砷对作物生长胁迫的相关研究仍相对较少。Tripathi等[14]研究发现,接种里氏木霉菌明显促进了土壤中砷的甲基化,降低了高毒性的无机态砷含量,减少了砷对作物生长的毒性胁迫;Su等[11]研究表明,棘孢木霉菌可通过影响砷在土壤中的形态分布特征,改变土壤中砷的有效性。
本文以棘孢木霉菌厚垣孢子为供试材料,通过盆栽试验研究接种棘孢木霉菌对砷污染土壤上小油菜生物量、砷吸收量、抗氧化胁迫相关酶活性,以及土壤砷有效性及化学形态等的影响,探讨了接种棘孢木霉菌调控土壤砷对小油菜生长胁迫的可能机理,相关结果可为今后砷污染农田的安全利用提供材料与技术支持。
1 材料与方法 1.1 试验材料土壤采自湖南省石门县白云乡鹤山村,土壤类型为第四纪红土母质发育的红壤,总砷含量为9.9 mg·kg-1,基本理化性质如表 1所示。土壤经自然风干后过2 mm筛待用。采用外源添加法制备砷污染土,外源砷为Na3AsO4·12H2O(北京中联化工试剂厂,分析纯)。研究采用的棘孢木霉厚垣孢子粉剂为课题组从湖南省石门县砷污染矿区分离出的耐砷真菌棘孢木霉菌(T.asperellum SM-12F1),经产孢培养制成厚垣孢子粉剂,具体过程见Wang等[15]。该菌株对砷具有高耐性、高积累和砷形态转化能力[11, 16]。供试作物为国家蔬菜工程技术研究中心培育的“夏绿2号”小油菜品种(Brassica campestris L.),生育期为40 d。
室内盆栽试验设置3个处理,分别为CK、AS、AS+CH。其中CK为空白对照,即采集的清洁土;AS为外源添加砷处理,具体过程为风干后的供试土壤以Na3AsO4·12H2O水溶液的方式加入外源砷(在前期试验的基础上,设置小油菜砷胁迫处理的土壤砷含量为120 mg·kg-1);AS+CH为添加相同量的外源砷后接种棘孢木霉孢子粉剂的处理,孢子粉剂的添加量为2%(W/W)。为了保证植物生长所需N、P、K等营养元素,实验前向土壤中施入尿素、磷酸二氢钾和硫酸钾,使其浓度分别为:0.72、0.32 g·kg-1和0.54 g·kg-1。各处理土壤混合均匀后装盆,每盆装土2.0 kg,各处理设置5个重复。各处理土壤稳定7 d后开始播种小油菜。盆栽试验于2017年8月—9月在中国农业科学院温室进行。试验期间采用称重法保持含水量为田间持水量的70%。盆栽40 d后,收获小油菜植株和土壤样品。
收获的小油菜植株样品测量株高、地上部和地下部生物量,将植株样品分为两部分:一部分样品于90 ℃杀青后,于60 ℃烘至恒重,研磨过0.149 mm筛用于总砷含量分析;另一部分放于-80 ℃冰箱冷冻保存,用于植株酶活性和砷化学形态分析。采集的土壤一部分经自然风干后,用于土壤pH、有效态砷含量等指标分析;另外一部分用于棘孢木霉活性菌落计数和砷化学形态分析。
1.3 样品分析方法土壤pH值测定采用水土比2.5:1水浸-电位法[17];有效态砷采用0.5 mol·L-1的NaHCO3溶液提取[18],用氢化物发生-原子荧光仪(HG-AFS,AFS-230,北京吉天仪器有限公司)测定;砷形态分析用超纯水-超声震荡提取法进行[19],取研磨过0.149 mm筛的自然风干土样1 g于离心管中,加入10 mL超纯水,超声震荡30 min,离心后过0.22 μm滤膜,采用高效液相色谱-电感耦合等离子体质谱仪(HPLC-ICP-MS,Agilent 8800,安捷伦科技有限公司)测定;供试土壤基本理化性状分析参见《土壤农化分析》[20];采用稀释平板涂布法获得棘孢木霉菌在土壤中的定殖数量,分离培养基为该菌株的专用选择性培养基[15]。本研究中,棘孢木霉菌在砷污染土壤中定殖情况良好,接种40 d后采集鲜土进行稀释平板涂布,接菌处理的土壤中棘孢木霉活菌数量可达9.3×107 cfu·g-1鲜土。
植物样品总砷含量采用HNO3-HClO4消解-原子荧光法测定[21];植物地上部砷形态的提取采用磷酸-超声辅助提取法[22],小油菜冷冻保存的鲜样用液氮冷冻研磨,称取研磨后的样品1 g于离心管中,加入10 mL 1%(V/V)的磷酸,超声震荡30 min,离心后过0.22 μm滤膜,利用HPLC-ICP-MS测定样品中砷形态。此外,为研究小油菜富集与转运砷的能力,本研究引入了砷生物富集系数(BCF)和转移系数(TF)。其中生物富集系数指小油菜体内的砷含量(包括地上和地下部)与土壤中砷含量的比值,转移系数指小油菜地上部砷含量与地下部砷含量的比值[23]。
抗逆相关酶活性和抗氧化物质含量分析:称取冷冻研磨后的小油菜地上部样品1 g于离心管中,加入5 mL 50 mmol·L-1磷酸缓冲液(PBS,pH 7.4)震荡30 min,4 ℃下12 000 g离心10 min,取上清液为粗酶提取液,于-80 ℃保存。超氧化物歧化酶(SOD)和过氧化物酶(POD)的测定参照Li等[24]的方法;过氧化氢酶(CAT)的测定以H2O2为底物,参照Aebi[25]的方法;还原型谷光甘肽(GSH)和抗坏血酸(AsA)的测定参照Wang等[26]的方法;丙二醛(MDA)的测定采用硫代巴比妥酸(TBA)比色法,参照王文银等[27]的研究。
1.4 数据处理采用Excel和Origin 9.0软件进行数据处理。采用SPSS 19.0对数据进行方差分析,处理间多重比较方法为Duncan法,差异显著性水平为P < 0.05。所有数据表示为平均值±标准误。
2 结果与分析 2.1 接种棘孢木霉菌对小油菜生物量及砷含量的影响接种棘孢木霉菌可显著促进污染土壤上小油菜生长,降低砷对其生长的胁迫影响(表 2)。与CK相比,AS处理小油菜株高显著降低了21.4%,地上部鲜重显著降低了26.2%,地下部鲜重显著降低了26.0%。与AS处理相比,AS+CH处理的小油菜株高显著增加了57.1%,地上部鲜重显著增加了81.8%,地下部鲜重显著增加了104.1%。

|
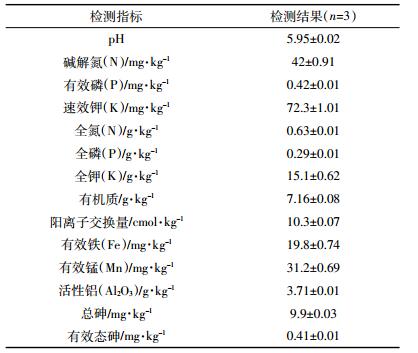
接种棘孢木霉菌可降低小油菜对砷的吸收(表 3)。CK处理小油菜地上部和地下部砷含量分别为0.5 mg·kg-1和1.15 mg·kg-1。添加外源砷显著增加了小油菜体内砷含量,AS处理小油菜地上部和地下部砷含量分别为5.79 mg·kg-1和23.55 mg·kg-1。接种棘孢木霉菌降低了小油菜对砷的吸收,AS+CH处理中小油菜砷含量较AS处理显著降低,地上部和地下部分别显著降低了12.4%和20.2%。此外,接种棘孢木霉厚垣孢子粉剂显著改变了小油菜对砷的BCF值,与AS处理相比,AS+CH处理中小油菜的BCF显著降低了7.8%,而TF值无显著差异。
|
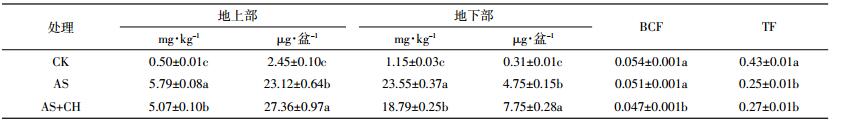
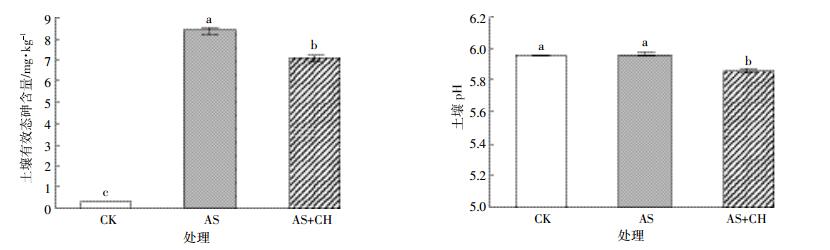
接种棘孢木霉菌降低了土壤有效态砷含量及土壤pH(图 1)。从有效态砷含量变化来看,CK处理的有效态砷含量最低,为0.31 mg·kg-1,添加外源砷处理的土壤有效态砷含量达到8.39 mg·kg-1,接种棘孢木霉菌降低了土壤有效态砷含量,与AS处理相比,AS+CH土壤有效态砷含量显著降低了15.7%。CK和AS处理的土壤pH无显著性差异,均为5.95,而AS+CH处理中土壤pH降为5.85。这可能是棘孢木霉菌在生长代谢过程中分泌有机酸类物质所导致,而土壤pH降低也是土壤有效态砷含量下降的可能原因之一。
|
| 不同小写字母表示不同处理在P<0.05水平差异显著 The different lowercase letters indicate significant difference among different treatments (P<0.05) 图 1 接种棘孢木霉菌后污染土壤中有效态砷含量和pH变化 Figure 1 The contents of available As and pH in soils inoculated with T.asperellum SM-12F1 |
接种棘孢木霉菌有效促进了污染土壤中砷的甲基化(图 2)。CK和AS处理土壤砷形态主要以As(Ⅴ)为主,同时伴随少量的As(Ⅲ),接种棘孢木霉菌后,土壤中除了As(Ⅴ)和As(Ⅲ)以外,同时检测到少量的一甲基砷(MMA)和二甲基砷(DMA)。与AS相比,AS+CH处理中4种形态砷总量降低了15.9%,这与土壤中有效态砷含量的变化趋势是一致的。AS+CH处理中MMA和DMA含量分别为0.075 mg·kg-1和0.048 mg·kg-1,分别占4种形态砷总量的3.8%和2.5%,As(Ⅲ)和As(Ⅴ)含量较AS处理分别显著降低18.8%和22%,在4种形态砷总量中的占比也分别由AS处理的3.1%和96.9%降低为AS+CH处理的2.9%和90.3%。以上结果表明,接种棘孢木霉菌显著促进了土壤中无机砷向有机砷的转化,降低了土壤中砷的毒性。
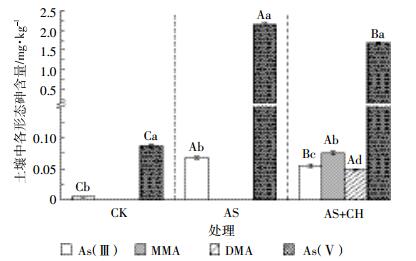
|
| 不同小写字母表示相同处理的不同形态砷含量间差异显著(P<0.05),不同大写字母表示相同砷形态在不同处理间差异显著(P<0.05)。下同 The different lowercase letters indicate the significant difference(P<0.05) in contents of different As speciations in the same treatment.The different uppercase letters indicate the significant difference(P<0.05) in contents of the same As speciation among different treatments.The same below 图 2 接种棘孢木霉菌后污染土壤中不同形态砷含量变化 Figure 2 The contents of As(Ⅲ), As(Ⅴ), MMA, and DMA in soils after inoculated with T.asperellum SM-12F1 |
接种棘孢木霉菌后,小油菜地上部砷形态变化与土壤砷形态保持一致,即均以As(Ⅴ)为主,其次为As(Ⅲ)、MMA和DMA(图 3)。接种棘孢木霉菌使小油菜地上部4种砷形态总量降低,与AS处理(2.30 mg·kg-1)相比显著降低了12.2%,该结果与小油菜体总砷含量变化一致(表 3),As(Ⅲ)和As(Ⅴ)含量分别显著降低了12.4%和15.6%。AS+CH处理MMA和DMA含量分别为0.048 mg·kg-1和0.028 mg·kg-1,分别占该处理4种形态砷总量的2.4%和1.4%。

|
| 图 3 接种棘孢木霉菌后污染土壤上小油菜地上部不同形态砷含量变化 Figure 3 The contents of As(Ⅲ), As(Ⅴ), MMA, and DMA in the shoot of Brassica campestris L.grown in As-contaminated soils inoculated with T.asperellum SM-12F1 |
外源砷提高了小油菜抗氧化酶活性和抗氧化物质含量,而接种棘孢木霉菌则降低了部分抗氧化酶和抗氧化物质含量(图 4)。各处理中,小油菜抗氧化酶SOD、POD和CAT活性及GSH、MDA和AsA含量均为CK最低。与CK相比,AS处理中SOD和CAT活性分别显著提高了92.3%和159%。接种棘孢木霉菌显著降低了小油菜植株内SOD和CAT活性。与AS处理相比,AS+CH处理中SOD和CAT活性分别显著降低了22.7%和41.1%。各处理间POD活性无显著差异。
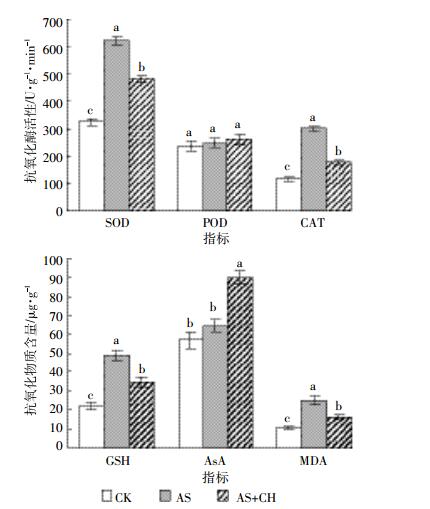
|
| 不同小写字母表示不同处理在P<0.05水平差异显著 The different lowercase letters indicate the significant difference among different treatments(P<0.05) 图 4 接种棘孢木霉菌后砷污染土壤上小油菜地上部抗氧化相关酶活性变化 Figure 4 The activities of antioxidant enzymes in the shoot of Brassica campestris L.grown in As-contaminated soils inoculated with T.asperellum SM-12F1 |
外源砷添加使小油菜体内MDA含量显著增加,较CK增加了131.6%。两种抗氧化物质GSH和AsA对外源砷的响应有所不同,AS处理中GSH含量较CK显著增加了122.1%,而AsA含量与CK相比无显著差异。接种棘孢木霉后小油菜体内GSH和MDA含量显著降低,较AS处理分别显著降低了29.2%和35.9%,而AsA含量则显著增加。
3 讨论本研究中外源砷添加浓度为120 mg·kg-1时,小油菜生长与不加砷的对照相比明显受到了抑制,具体表现为小油菜生物量显著下降,抗氧化系统如SOD和CAT活性、GSH和MDA含量显著提升。接种棘孢木霉菌显著减缓了土壤中砷对小油菜生长的胁迫,提高了小油菜生物量,降低了小油菜体内部分抗氧化酶活性或抗氧化物质含量。已有结果已经证明,棘孢木霉菌能够溶解土壤磷,从而增加土壤有效磷含量,能够释放氨基环丙烷-羧酸(ACC)脱氨酶、生长素和嗜铁素等,促进作物生长[28]。本研究中棘孢木霉菌对小油菜生长的促进,有助于缓解土壤砷胁迫;接种棘孢木霉后显著降低了小油菜体内砷的浓度,有助于降低小油菜体内砷的毒性。小油菜体内砷浓度下降除了与土壤有效态砷变化相关外,也与其生物稀释作用有关。接种棘孢木霉菌提高了小油菜生物量,有助于小油菜体砷浓度的降低;接种棘孢木霉能够促进土壤无机态砷向有机态砷转化并影响小油菜地上部各形态砷含量,降低土壤和小油菜体内砷的毒性。已有研究表明,环境中As(Ⅲ)毒性显著高于As(Ⅴ),而无机砷的毒性显著大于有机砷[29]。本研究中接种棘孢木霉菌促进了土壤和小油菜体内无机砷向有机砷转化,且与AS处理相比,显著降低了As(Ⅲ)的含量和比例。该结果与Tripathi等[14, 30]的研究结果具有一致性;接种棘孢木霉菌显著降低了土壤有效态砷的含量,进而减少了小油菜对砷的吸收,减缓了砷对小油菜生长的胁迫。Maheswari等[31]的研究认为,真菌细胞壁上丰富的羟基、羧基和多糖等物质可以吸附固定砷,降低其生物可利用性,这可能是本研究中棘孢木霉菌降低土壤砷有效性的可能原因之一。土壤pH变化也显著影响着土壤砷的有效性[32],真菌的代谢产物中往往含有各类有机酸,这些物质不仅可以与土壤中游离态的砷络合,还可以通过降低土壤pH增加砷在土壤胶体表面的吸附[33]。
从小油菜地上部各形态砷含量变化来看,接种棘孢木霉菌使小油菜地上部无机砷含量及占总量的比例下降,有机砷含量及其比例提高。该结果亦与土壤中砷形态变化一致。一般认为,作物体内As(Ⅲ)毒性最高,而有机砷和As(Ⅴ)的毒性较低,这是因为MMA和As(Ⅴ)能诱导作物体内合成非蛋白巯醇类物质(NPTs)[34],而MMA和As(Ⅴ)与该类物质的络合被认为是砷在作物体内重要的解毒机制[35]。可见,接种棘孢木霉菌能够改变土壤中有效态砷含量及其形态特征,进而降低小油菜对砷的吸收,减缓砷对小油菜生长的毒性。
氧化胁迫是砷对作物生长的重要毒性作用机制[36]。SOD最先参与植物抗氧化反应,可将O2-歧化为毒性较弱的H2O2和O2,H2O2可通过POD和CAT的作用来清除[37-38]。本研究中,外源添加高浓度砷使小油菜地上部SOD和CAT的活性显著提高,可以认为是小油菜受到砷胁迫的表现。这与郝玉波等[3]的研究结果一致。接种棘孢木霉菌使小油菜地上部抗氧化酶如SOD和CAT活性降低,表明砷胁迫作用下降。MDA是植物膜脂过氧化的主要产物,其含量的变化可在一定程度上反映植物体受重金属氧化胁迫的水平[39]。本研究中,小油菜体内MDA含量的变化与SOD和CAT的变化一致。外源砷增加了小油菜体内氧化胁迫,使MDA含量升高,接种棘孢木霉菌减缓了外源砷对小油菜生长的氧化胁迫,降低了MDA含量。此外,AsA-GSH循环是清除植物体内过多H2O2重要途径[40],同时,GSH是合成植物金属螯合肽(PCs)的直接前体[41]。本研究中,接种棘孢木霉菌减低了小油菜体内砷的含量,从而导致小油菜体内GSH响应量下降。而小油菜体内AsA含量变化与MDA和GSH不一致,其原因还需进一步分析。
4 结论(1)土壤中外源砷浓度120 mg·kg-1条件下小油菜生长明显受到抑制,而接种棘孢木霉菌显著减缓了土壤砷对小油菜生长的胁迫,促进其生物量增加。
(2)接种棘孢木霉菌在一定程度上降低了土壤有效态砷含量,同时减少了小油菜对砷的吸收。接种棘孢木霉菌促进了土壤砷由无机态向有机态转化,显著影响了小油菜地上部砷的形态特征,在一定程度上降低了小油菜体内砷的毒性。
(3)外源砷胁迫下小油菜抗氧化胁迫相关酶活性显著升高,而接种棘孢木霉菌后小油菜体内部分相关酶活性下降。接种棘孢木霉菌可有效缓解砷对小油菜的氧化胁迫。
| [1] |
李仁英, 沈孝辉, 张耀鸿, 等. 无机砷对不同水稻品种种子萌发和幼苗光合生理的影响[J]. 农业环境科学学报, 2014, 33(6): 1067-1074. LI Ren-ying, SHEN Xiao-hui, ZHANG Yao-hong, et al. Effects of inorganic arsenic on seed germination and photosynthetic characteristics of various rice cultivars[J]. Journal of Agro-Environment Science, 2014, 33(6): 1067-1074. DOI:10.11654/jaes.2014.06.002 (in Chinese) |
| [2] |
Tripathi R D, Srivastava S, Mishra S, et al. Arsenic hazards:Strategies for tolerance and remediation by plants[J]. Trends in Biotechnology, 2007, 25(4): 158-165. DOI:10.1016/j.tibtech.2007.02.003 |
| [3] |
郝玉波, 刘华琳, 慈晓科, 等. 砷对玉米生长、抗氧化系统及离子分布的影响[J]. 应用生态学报, 2010, 21(12): 3183-3190. HAO Yu-bo, LIU Hua-lin, CI Xiao-ke, et al. Effects of arsenic on maize growth, antioxidant system and ion distribution[J]. Chinese Journal of Applied Ecology, 2010, 21(12): 3183-3190. (in Chinese) |
| [4] |
Chakrabarty D, Trivedi P K, Misra P, et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings[J]. Chemosphere, 2009, 74(5): 688-702. DOI:10.1016/j.chemosphere.2008.09.082 |
| [5] |
安堃达, 熊双莲, 涂书新, 等. 豇豆和番茄对砷胁迫的响应[J]. 华中农业大学学报, 2013, 32(1): 73-77. AN Kun-da, XIONG Shuang-lian, TU Shu-xin, et al. Arsenic stressed response of cowpea and tomato[J]. Journal of Huazhong Agricultural University, 2013, 32(1): 73-77. (in Chinese) |
| [6] |
马茹茹, 卜玉山, 史晓凯. 施肥和外源砷对小白菜生长和抗性生理的影响[J]. 植物营养与肥料学报, 2012, 18(5): 1213-1219. MA Ru-ru, BU Yu-shan, SHI Xiao-kai. Effects of fertilization and exogenous arsenic on resistance physiology and growth of pak-choi[J]. Plant Nutrition and Fertilizer Science, 2012, 18(5): 1213-1219. (in Chinese) |
| [7] |
张敏, 孙宇, 冯宇佳, 等. 硅促进水稻种子萌发及缓解幼苗砷毒性的效应研究[J]. 生态毒理学报, 2017, 12(1): 243-250. ZHANG Min, SUN Yu, FENG Yu-jia, et al. Silicon treatments accelerated rice seed germination and mitigated arsenic toxicity to seedlings[J]. Asian Journal of Ecotoxicology, 2017, 12(1): 243-250. DOI:10.7524/AJE.1673-5897.20160503002 (in Chinese) |
| [8] |
赵秀峰, 张强, 程滨, 等. 硒对砷胁迫下小白菜生理特性及砷吸收的影响[J]. 环境科学学报, 2017, 37(9): 3583-3589. ZHAO Xiu-feng, ZHANG Qiang, CHENG Bin, et al. Effects of selenium on the physiological characteristics and As uptake of pak choi under As stress[J]. Acta Scientiae Circumstantiae, 2017, 37(9): 3583-3589. (in Chinese) |
| [9] |
余天红, 黎华寿. 砷污染土壤微生物修复机制及其研究进展[J]. 环境污染与防治, 2014, 36(12): 77-82. YU Tian-hong, LI Hua-shou. Mechanism of bioremediation in arsenic contaminated soil and its research progress[J]. Environmental Pollution & Control, 2014, 36(12): 77-82. DOI:10.3969/j.issn.1001-3865.2014.12.017 (in Chinese) |
| [10] |
Singh M, Srivastava P K, Verma P C, et al. Soil fungi for mycoremediation of arsenic pollution in agriculture soils[J]. Journal of Applied Microbiology, 2015, 119(5): 1278-1290. DOI:10.1111/jam.2015.119.issue-5 |
| [11] |
Su S M, Zeng X B, Bai L Y, et al. Inoculating chlamydospores of Trichoderma asperellum SM-12F1 changes arsenic availability and enzyme activity in soils and improves water spinach growth[J]. Chemosphere, 2017, 175: 497-504. DOI:10.1016/j.chemosphere.2017.02.048 |
| [12] |
Babu A G, Shim J, Bang K S, et al. Trichoderma virens PDR-28:A heavy metal-tolerant and plant growth promoting fungus for remediation and bioenergy crop production on mine tailing soil[J]. Environmental Management, 2014, 132: 129-134. |
| [13] |
刘云霞, 周益奇, 董妍, 等. 接种丛枝菌根真菌(Glomus mosseae)对旱稻吸收砷及土壤砷形态变化的影响[J]. 生态毒理学报, 2012, 7(2): 195-200. LIU Yun-xia, ZHOU Yi-qi, DONG Yan, et al. Effect of inoculation of arbuscular mycorrhizal fungi(Glomus mosseae)on As uptake of upland rice and transformation of As speciation in soil[J]. Asian Journal of Ecotoxicology, 2012, 7(2): 195-200. (in Chinese) |
| [14] |
Tripathi P, Singh P C, Mishra A, et al. Trichoderma inoculation augments grain amino acids and mineral nutrients by modulating arsenic speciation and accumulation in chickpea(Cicer arietinum L.)[J]. Ecotoxicology and Environmental Safety, 2015, 117: 72-80. DOI:10.1016/j.ecoenv.2014.10.027 |
| [15] |
Wang X R, Su S M, Zeng X B, et al. Inoculation with chlamydospores of Trichoderma asperellum SM-12F1 accelerated arsenic volatilization and influenced arsenic availability in soils[J]. Journal of Integrative Agriculture, 2014, 14(2): 389-397. |
| [16] |
Zeng X B, Su S M, Jiang X L, et al. Capability of pentavalent arsenic bioaccumulation and biovolatilization of three fungal strains under laboratory conditions[J]. Clean-Soil Air Water, 2010, 38(3): 238-241. DOI:10.1002/(ISSN)1863-0650 |
| [17] |
马庆旭, 王峻, 曹小闯, 等. 土壤pH对玉米与微生物竞争吸收氨基酸的影响[J]. 应用生态学报, 2017, 28(7): 2277-2284. MA Qing-xu, WANG Jun, CAO Xiao-chuang, et al. Effects of soil pH on the competitive uptake of amino acids by maize and microorganisms[J]. Chinese Journal of Applied Ecology, 2017, 28(7): 2277-2284. (in Chinese) |
| [18] |
Woolson E A, Axley J H, Prohaska T, et al. Correlation between available soil arsenic, estimated by six methods, and response of corn(Zea mays L.)[J]. Soil Science Society of America Journal, 1971, 35: 101-105. DOI:10.2136/sssaj1971.03615995003500010030x |
| [19] |
杨芬, 韦朝阳, 刘金鑫. 砷形态分析的样品前处理技术研究进展[J]. 环境科学与技术, 2016, 39(10): 79-86. YANG Fen, WEI Chao-yang, LIU Jin-xin. A review on sample pretreatment techniques of arsenic speciation analysis[J]. Environmental Science & Technology, 2016, 39(10): 79-86. (in Chinese) |
| [20] |
鲁如坤. 土壤农业化学分析方法[M]. 北京: 中国农业科技出版社, 2000. LU Ru-kun. Analytical methods of soil and agricultural chemistry[M]. Beijing: China Agricultural Science and Technology Press, 2000. (in Chinese) |
| [21] |
Bergqvist C, Herbert R, Persson I, et al. Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables[J]. Environmental Pollution, 2014, 184: 540-546. DOI:10.1016/j.envpol.2013.10.003 |
| [22] |
倪张林, 汤富彬, 屈明华, 等. 高效液相色谱-电感耦合等离子体质谱联用技术测定花茶中砷形态[J]. 分析科学学报, 2013, 29(4): 506-510. NI Zhang-lin, TANG Fu-bin, QU Ming-hua, et al. Determination of arsenic species in scented tea by HPLC-ICP-MS[J]. Journal of Analytical Science, 2013, 29(4): 506-510. (in Chinese) |
| [23] |
余轲, 刘杰, 尚伟伟, 等. 青葙对土壤锰的耐性和富集特征[J]. 生态学报, 2015, 35(16): 5430-5436. YU Ke, LIU Jie, SHANG Wei-wei, et al. Tolerance and accumulation characteristics of Celosia argentea Linn.growing in Mn-contaminated soil[J]. Acta Ecologica Sinica,, 2015, 35(16): 5430-5436. (in Chinese) |
| [24] |
Li J X, Dai X M, Li L Y, et al. Metabolism of reactive oxygen species in cytoplasmic male sterility of rice by marking upmost pulvinus interval[J]. Applied Biochemistry and Biotechnology, 2015, 175(3): 1263-1269. DOI:10.1007/s12010-014-1346-8 |
| [25] |
Aebi H. Catalase in vitro[J]. Methods Enzymol, 1984, 105: 121-126. DOI:10.1016/S0076-6879(84)05016-3 |
| [26] |
Wang J L, Zeng Q, Zhu J G, et al. Dissimilarity of ascorbate-glutathione (AsA-GSH)cycle mechanism in two rice (Oryza sativa L.)cultivars under experimental free-air ozone exposure[J]. Agriculture Ecosystems & Environment, 2013, 165: 39-49. |
| [27] |
王文银, 高小刚, 司晓林, 等. 外源钙盐对盐胁迫下沙拐枣渗透调节和膜脂过氧化的影响[J]. 环境科学研究, 2017, 30(8): 1230-1237. WANG Wen-yin, GAO Xiao-gang, SI Xiao-lin, et al. Effects of exogenous calcium on osmotic adjustment and peroxidation of Calligonum mongolicum membrane under salt stress[J]. Research of Environmental Sciences, 2017, 30(8): 1230-1237. (in Chinese) |
| [28] |
Qi W, Zhao L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress[J]. Journal of Basic Microbiology, 2013, 53: 355-364. DOI:10.1002/jobm.v53.4 |
| [29] |
刘艳丽, 徐莹, 杜克兵, 等. 无机砷在植物体内的吸收和代谢机制[J]. 应用生态学报, 2012, 23(3): 842-848. LIU Yan-li, XU Ying, DU Ke-bing, et al. Absorption and metabolism mechanisms of inorganic arsenic in plants:A review[J]. Chinese Journal of Applied Ecology, 2012, 23(3): 842-848. (in Chinese) |
| [30] |
Tripathi P, Singh P C, Mishra A, et al. Arsenic tolerant Trichoderma sp.reduces arsenic induced stress in chickpea (Cicer arietinum)[J]. Environmental Pollution, 2017, 223: 137-145. DOI:10.1016/j.envpol.2016.12.073 |
| [31] |
Maheswari S, Murugesan A G. Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic contaminated site[J]. Environmental Technology, 2009, 30(9): 921-926. DOI:10.1080/09593330902971279 |
| [32] |
钟松雄, 尹光彩, 陈志良, 等. Eh、pH和铁对水稻土砷释放的影响机制[J]. 环境科学, 2017, 38(6): 2530-2537. ZHONG Song-xiong, YIN Guang-cai, CHEN Zhi-liang, et al. Influencing mechanism of Eh, pH and iron on the release of arsenic in paddy soil[J]. Environmental Science, 2017, 38(6): 2530-2537. (in Chinese) |
| [33] |
高雪, 曾希柏, 白玲玉, 等. 有机酸对As (Ⅴ)在土壤中老化的影响[J]. 农业环境科学学报, 2017, 36(8): 1526-1536. GAO Xue, ZENG Xi-bai, BAI Ling-yu, et al. Influence of organic acids on As(Ⅴ) aging in soils[J]. Journal of Agro-Environment Science, 2017, 36(8): 1526-1536. DOI:10.11654/jaes.2017-0189 (in Chinese) |
| [34] |
Tang Z, Kang Y, Wang P, et al. Phytotoxicity and detoxification mechanism differ among inorganic and methylated arsenic species in Arabidopsis thaliana[J]. Plant Soil, 2016, 401: 243-257. DOI:10.1007/s11104-015-2739-3 |
| [35] |
Mishra S, Mattusch J, Wennrich R. Accumulation and transformation of inorganic and organic arsenic in rice and role of thiol complexation to restrict their translocation to shoot[J]. Scientific Reports, 2017, 7: e40522. DOI:10.1038/srep40522 |
| [36] |
赖长鸿, 刘亚玲, 贺鸿志, 等. 无机三价砷对生菜的生态毒性及其生物积累[J]. 农业环境科学学报, 2015, 34(5): 831-836. LAI Chang-hong, LIU Ya-ling, HE Hong-zhi, et al. Eco-toxicity and accumulation of inorganic trivalent arsenic in lettuce[J]. Journal of Agro-Environment Science, 2015, 34(5): 831-836. DOI:10.11654/jaes.2015.05.003 (in Chinese) |
| [37] |
Wu L, Yi H, Yi M. Assessment of arsenic toxicity using Allium/Vicia root tip micronucleus assays[J]. Journal of Hazardous Materials, 2010, 176(1-3): 952-956. DOI:10.1016/j.jhazmat.2009.11.132 |
| [38] |
杨牟, 何平, 段才绪, 等. 外源SNP, Spd对盐碱胁迫下射干幼苗体内抗氧化酶活性的影响[J]. 西南大学学报(自然科学版), 2015, 37(6): 13-19. YANG Mou, HE Ping, DUAN Cai-xu, et al. Effects of exogenous SNP and Spd on the activity of antioxidant enzyme system in Belamacanda chinensis(L.)DC.seedlings under saline-alkali stress[J]. Journal of Southwest University(Natural Science Edition), 2015, 37(6): 13-19. (in Chinese) |
| [39] |
Khoshgoftarmanesh A H, Khodarahmi S, Haghighi M, et al. Effect of silicon nutrition on lipid peroxidation and antioxidant response of cucumber plants exposed to salinity stress[J]. Archives of Agronomy & Soil Science, 2013, 60(5): 639-653. |
| [40] |
周翠, 张雯, 王婷, 等. 秋华柳抗氧化酶系统对镉胁迫的响应[J]. 西南大学学报(自然科学版), 2017, 39(10): 27-34. ZHOU Cui, ZHANG Wen, WANG Ting, et al. Response of the antioxidant enzyme system of Salix variegata franch to cadmium stress[J]. Journal of Southwest University(Natural Science Edition), 2017, 39(10): 27-34. (in Chinese) |
| [41] |
Ha S B, Smith A P, Howden R, et al. Phytochelatin synthase genes from arabidopsis and the yeast Schizosaccharomyces pombe[J]. The Plant Cell, 1999, 11: 1153-1163. DOI:10.1105/tpc.11.6.1153 |
 2018, Vol. 35
2018, Vol. 35