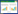文章信息
- 孙凯, 李舜尧
- SUN Kai, LI Shun-yao
- 接种功能内生细菌降低作物体内POPs污染
- Feasibility Analysis for Mitigating the Contamination of POPs in Crops Through Inoculation with Functional Endophytic Bacteria
- 农业资源与环境学报, 2017, 34(5): 397-404
- Journal of Agricultural Resources and Environment, 2017, 34(5): 397-404
- http://dx.doi.org/10.13254/j.jare.2017.0080
-
文章历史
- 收稿日期: 2017-03-24
2. 南京农业大学资源与环境科学学院, 江苏 南京 210095
2. College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing 210095, China
“万物土中生”,土壤是环境核心介质,是动物和人群存活根本。由于工农业生产的迅猛发展,大量持久性有机污染物(Persistent organic pollutants,POPs)进人农业土壤环境系统并持续积累,引起土壤化学性质、有机质含量和微生物区系等发生明显改变,导致土壤环境总体质量趋于下降,进而对我国农业生态安全和可持续发展造成严重的危害[1]。POPs释放到农业土壤环境中,不仅可以被农作物摄取积累导致粮食减产,也能够通过食物链传递危害动物和人群健康[2-3]。虽然,关于POPs引发的动物和人群健康风险危害多与POPs污染事故相关而非常态,但是,厘清农业土壤环境中POPs在土壤-植物之间的归趋和转化有助于解决POPs污染造成的农作物体内有机污染残留问题。
目前,国内外研究者已经建立了平衡模型、动力学模型和稳态模型等用于预测和评估植物吸收、积累和代谢POPs的功效[4-6]。普遍认为,POPs从土壤进人植物体系主要包括2种途径:即根系吸收和茎叶吸收。根系从土壤固相中吸收解吸POPs, 并随蒸腾拉力作用沿木质部向地上部位传导,其对亲脂性POPs的吸收与其辛醇-水分配系数、根脂肪含量呈正相关性,与土壤有机质含量呈负相关性[5-6]。茎叶可以直接吸收大气中POPs,并通过扩散作用分配到植物亚细胞微区,其吸收强度主要取决于POPs的辛醇-气分配系数、亨利系数、茎叶比表面积和脂肪含量[7]。因此,植物摄取POPs可看作是其在土壤-溶液-植物等各相介质中连续分配作用的总和[4]。已有资料显示,植物吸收POPs后能够通过功能化、轭合和区室化等作用降低POPs对自身的毒害作用[8]; 但是,仍然有大量的POPs残留在植物体内[9]。因此,采用何种技术手段调控植物对POPs的吸收积累和降解代谢,从而有效地规避植物体内POPs污染残留、加强农业食品安全评价仍然是农业生态领域亟需解决的难点问题之一。
近年来,诸多研究表明植物微生态系统中含有数量和种类丰富的功能内生细菌(Endophytic bacteria,EB),其能重新定殖在目标植物的组织间隙或细胞内部,促进植物生长、提高植物对有害环境的抵抗性[10-11]。能否利用植物-功能EB的微生态系统,从污染厂区的健康植物体内分离、筛选出具有POPs降解特性的功能EB,并将其重新定殖在目标农作物体内,进而有效地去除作物体内POPs、规避作物污染风险已经引起国内外学者的广泛关注。与从其他环境介质中分离的功能菌对比,从污染区植物体内筛选的具有POPs降解功能的EB能够更好地适应植物体内环境、有可能良好定殖并发挥降解效能。本文综述了农业土壤环境中作物体内POPs的污染现状及植物细胞对POPs的吸收转运和代谢机理,系统地阐释了功能EB的最新研究进展,剖析了功能EB与宿主植物特殊的生态关系,综合地评价了利用植物-功能EB的微生态系统解决农作物体内POPs污染残留的技术体系,以期为指导污染区粮食安全生产、规避作物POPs污染风险、保障食品安全和重新利用污染耕地资源等提供理论依据和技术支撑。
1 植物对POPs的吸收 1.1 植物POPs污染现状及危害植物可吸收积累农业生态系统中的POPs,并由食物网传递危害动物和人群健康。农业土壤环境中的POPs主要来源于农药化肥使用、农业废弃物燃烧和污水灌溉等,它们具有高毒、持久和难降解等特点[3, 12]。Ksmes等[13]调查了工业污染区土壤中多环芳烃(Poly-cyclic aromatic hydrocarbons,PAHs)进人作物体内的迁移和转化过程,结果发现,煤气厂土壤中PAHs呈梯度分布,浓度变化范围为4~2526 mg·kg-1,农作物能够摄取土壤中的PAHs并迁移到地上部位,其浓度变化范围为0.2~2.7 mg·kg-1。表 1列举了不同农作物体内POPs的污染残留状况。由表 1可知,农作物如青菜、水稻、西葫芦、南瓜和萝卜等对土壤中POPs均具有一定的摄取和积累能力,并可能通过食物网积聚,对生态农业和人类健康构成潜在的危害。已有资料表明,人类食用POPs严重超标的农产品后,可能引发急性中毒,而较少的POPs在人体内长期蓄积滞留也会引起诸多慢性疾病[20]。例如,POPs可在人体脂质、胚胎及肝脏等处积累,其毒理学危害主要包括对生物体的“三致”效应、干扰DNA转录和复制以及对生殖系统、内分泌系统和神经传导系统的损害等[21-22]。我国作为农作物生产和销售大国,因误食高浓度POPs污染的农产品而引发的人畜中毒现象逐年攀升,并因农产品中POPs残留量超标而导致农作物销售受阻。因此,如何有效地解决农产品中POPs的污染残留问题、保障农业的健康可持续发展亟待解决。
|
植物能够通过自我调控机制将POPs部分代谢或分配到亚细胞微区中。根系从土壤中吸收POPs后,其不仅可以穿过细胞壁、细胞膜进人细胞液和细胞器等亚细胞微区,也可以在植物蒸腾拉力作用下向植物茎叶传输[23]。植物茎叶吸收大气中的POPs需要通过气孔或表皮,该过程需要穿透气体和液体两个界面[8]。植物摄取POPs后,可以通过刺激植物细胞中一系列生物化学和生理学进程来降低POPs对自身的毒害作用[24]。该过程可以调控POPs在植物亚细胞微区中的分配行为,并通过木质化作用将其降解代谢为次生代谢产物固定在植物细胞内,或被植物细胞内的相关代谢酶系彻底矿化为CO2和H2O。
POPs在植物细胞内能够通过相关酶系的氧化、还原和水解等反应形成亲水性活性官能团,如羟基、羧基和氨基等。这些活性功能团化合物既可以被植物体内的相关酶系深度氧化为CO2和H2O (占整体0.1%~5%),又能够与植物细胞内的内源性化合物(如糖类、脂肪酸、蛋白质和氨基酸等)结合形成共价轭合产物[8]。该类轭合产物分为可溶性组分和不可溶性组分,它们通过区室化作用分别存储在植物液泡和细胞壁中。例如,Sterling和Balke[25]研究指出,细胞培养条件下大豆可将除草剂苯达松羟基化形成8-羟基-苯达松,其能够与葡萄糖结合形成共价耦合产物; 有机氯农药在植物体内羟基化后,能够与葡萄糖和丙二酰耦合残留在液泡中[26]。POPs在植物细胞内的氧化、转移主要由植物体内氧化酶、还原酶、酷酶和去卤化酶等多种代谢酶系共同控制。例如,谷胱甘肽-S-转移酶能够诱导POPs形成活性官能团化合物并与谷胱甘肽耦合,从而减轻POPs对植物自身的毒害作用[27]。由此可见,采用相关技术手段调控植物体内POPs的降解酶系活性,有望增强植物对POPs的代谢转移,从而降低植物体内POPs污染含量。
2 功能内生细菌功能EB作为植物微生态系统中的重要构成部件,具有特殊的生态作用和广谱功效,可能用于调控植物体内POPs的降解代谢[10, 28]。功能EB不仅能够通过自身的代谢作用产生生物活性物质(如吲哚乙酸、铁载体、细胞分裂素、乙烯和赤霉素等)刺激植物生长活动,也可以借助信号传导作用诱导植物体内相关酶系的活性、提高植物对营养物质的摄取和利用率[29]。此外,某些功能EB也能够增强植物的抗逆性、诱导植物产生防御系统抵抗病原菌侵害、甚至减少植物体内POPs的污染残留[10, 28]。作为回报,宿主植物也能够为功能EB提供稳定的生态区位和大量的营养物质,促进功能EB的生长繁殖[10]。鉴于植物-功能EB的协同交互作用,有望从POPs污染区的健康植物体内获得具有POPs降解特性的功能EB,并将其重新应用于目标农作物体内的定殖,从而提高作物产量、降低作物体内的POPs污染风险。
2.1 功能内生细菌的分离筛选及其在体外降解POPs研究者主要采用选择性富集培养方法,从POPs污染区的健康植物体内分离、筛选出具有POPs降解特性的功能EB[28, 30],并结合功能EB的形态学特征、生理生化特性和16SrRNA序列同源性分析,确定其分类学地位。目前,已分离的具有POPs降解特性的可培养功能EB主要包括假单胞菌属(Pseudomonas sp.)、红球菌属(Rhodococcus sp.)、肠杆菌属(Enterobacter sp.)、伯克氏菌属(Burkholderia sp.)和芽抱杆菌属(Bacillus sp.)等,这些功能EB具有广泛的开发和应用价值[10, 30-32]。例如,Zhu等[30]以PAH为碳源和能源,从小蓬草和三叶草体内分别获得1株具有PAHs降解效能的内生寡养单胞菌(Stenotrophomonas sp. P1)和假单胞菌P3, 证实植物可为功能EB提供一个稳定的生态区位。Sessitsch等[31]采用宏基因组学分析了水稻根中功能EB的多样性和作用功效,指出这些功能EB具有降解代谢脂肪族芳香烃的潜力。Phillips等[32]从植物修复污染区的多年生黑麦草体内获得多株具有PAH降解特性的功能EB,并采用14C标记测定了功能降解菌群对PAH的矿化作用,结果表明,该功能内生群落能够有效地减少根际土壤中PAH含量。
功能EB在植物体外代谢POPs的机制与环境微生物降解POPs相似。以PAHs为模式POPs,简单概述功能EB降解POPs的关键酶系、代谢产物和作用机理。其降解机理主要是在芳香环羟基化双加氧酶作用下,使PAHs苯环中2个相邻碳原子羟基化,形成顺-二醇,再经过多种酶系共同作用形成水杨酸或邻苯二甲酸,之后经过邻苯二酚途径进人三羧酸循环,最终彻底氧化分解为CO2和H2O[33]。功能EB代谢PAHs的过程与诸多降解基因或基因簇密切相关,这些基因能够通过编码PAHs的代谢酶系降解PAHs。研究已经证实,ahdA、bphA和PhnA等基因是鞘脂菌属(Sphingobium sp.)控制菲降解的关键基因[34-36]; nah基因簇是编码假单胞菌降解PAHs的一大类经典基因簇[37]。基于以上分析可知,从POPs污染区的健康植物体内分离、筛选出具有POPs降解功能的EB能够在宿主植物体外有效地降解POPs。能否将具有POPs降解功能的EB重新定殖在目标农作物体内,利用功能EB在作物体内的特殊生态区位和广谱功效,规避作物体内POPs的污染风险?该想法已经得到国内外研究者的广泛重视。
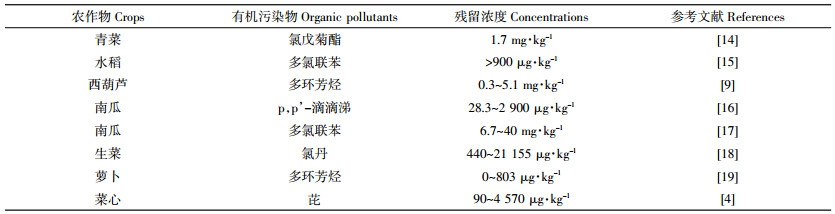
2.2 功能内生细菌降低目标植物体内POPs污染已有资料表明,从POPs污染区的健康植物体内筛选出具有POPs降解特性的功能EB,并将其重新定殖在目标植物体内能够发挥特殊功效[38-40]。与从土壤等介质中获得的POPs降解菌对比,从植物体内筛选的功能EB能够更好地适应植物内部的环境、有望良好定殖并发挥降解效能。目前,关于功能EB在目标植物体内定殖的研究方法主要包括浸种、灌根、伤根、喷洒、涂叶和注射叶腋等[10]。植物接种功能EB后,其不仅能够定殖在初始的接种部位,也可以通过扩散作用迁移到宿主植物的其他组织和器官内,主要定殖位点是细胞间隙和维管束导管[10]。采用绿色荧光蛋白(Green fluorescent protein, GFP)基因标记技术能够有效地探寻功能EB在目标植物体内的定殖动态和分布情况。GFP包含特殊的生色官能团,当其受到蓝光或紫光激发后,能量由Ca2+激活光蛋白产生肉眼可见的绿色荧光。如图 1所示,该技术提供了一种独特的可视化表现型,能够用于多方位追踪和定位功能EB在植物体内的定殖分布[38]。此外,荧光共振能量转移技术的应用也为检测功能EB在宿主植物细胞内部的定殖提供了新的追踪手段[41]。
|
| 图 1 功能内生假单胞菌株Ph6-gfp在黑麦草根、茎和叶中定殖分布 Figure 1 Endophytic colonization of Pseudomonas sp. Ph6-gfp observed via gfp tagging show in plants |
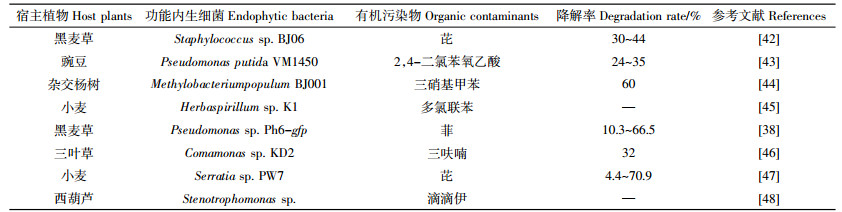
鉴于功能EB特殊的生态区位、良好的降解特性和优越的定殖效能等优点,从POPs污染的植物体内分离、筛选出功能EB并将其重新定殖在目标农作物体内,有望针对性地解决作物体内POPs的污染残留问题,从而生产出绿色无公害化的健康农产品[10, 28]。例如,Sun等[42]从PAHs污染的看麦娘体内获得1株芘降解功能内生葡萄球菌(Staphylococcus sp.BJ06);该菌不仅能够有效地定殖在植物体内促进植物生长,也可以降低植物体内的芘浓度和积累量。另有研究证实,将功能内生假单胞菌VM1450定殖到豌豆体内,该菌不仅可以规避2,4-二氯苯氧乙酸(2,4-D)对豌豆的毒害作用,也能够阻控豌豆对2, 4-D的吸收和积累[43]。表 2总结了功能EB重新定殖在目标植物体内对植物体内POPs降解代谢的影响。由表 2可知,具有POPs降解特性的功能EB能够重新定殖在目标植物体内,并有效地减少植物体内POPs残留。因此,植物-功能EB的微生态系统可能用于规避农作物体内POPs的污染风险,保障农产品安全和人群健康。功能EB促进宿主植物代谢POPs的效率与多种因素密切相关,如植物和功能EB种类、EB在植物体内的定殖数量和活性、植物对携带POPs降解基因的EB的选择能力和POPs的理化特性等降[28, 30, 32, 40]。
|
已有研究资料显示,功能EB降解代谢植物体内POPs的作用机理主要包括以下4点:
(1) 功能EB直接降解作物体内POPs。功能EB定殖在宿主植物的细胞间隙和维管束导管中,可以直接利用植物体内POPs作为唯一的碳源和能源进行生长繁殖,也能够以共代谢的方式降解代谢植物体内POPs[10, 38, 49]。例如,当缺乏共代谢酶诱导或维持细菌生长的底物浓度较低时,功能EB可以直接降解代谢宿主植物体内的某些POPs; 而当存在共代谢酶诱导时(由宿主植物的光合作用或二次代谢提供),功能EB能够利用植物体内的内源性有机物,以共代谢的方式增强其对植物体内POPs的降解效能,其降解机理与功能EB在植物体外代谢POPs类似[38, 50-51]。目前,可以结合DNA和稳定同位素标记技术鉴定功能EB调控的植物体内POPs的转化产物。
(2) 功能EB影响植物体内相关代谢酶系的活性。功能EB定殖在植物体内可能通过影响与植物体内POPs代谢相关的酶系活性,加快宿主植物和功能EB对POPs的降解代谢潜能[10, 52-53]。植物体内代谢POPs的关键酶系主要包括细胞色素P450酶、谷胱甘肽-S-转移酶、过氧化物酶、超氧化物歧化酶、多酚氧化酶、硝基还原酶、酷酶和水解酶等,它们可以直接参与植物体内POPs的诸多代谢反应,调控POPs在植物体内的含量和分布[8]。盛月慧等[54]研究指出,黑麦草体内不仅存在多种关键酶系能够氧化降解PAH,也存在数量可观的PAH降解功能EB; 当植物受到PAH污染胁迫时,茎叶中过氧化物酶和多酚氧化酶活性以及可培养功能EB均有所响应,从而介导植物对PAH的应答反应。Weyens等[39]认为接种功能EB能够影响植物体内接触酶和超氧化物歧化酶活性,促使植物积极应对污染物对自身的生物毒害作用。此外,功能EB也可以产生双加氧酶促进POPs的苯环裂解,直接代谢植物体内的POPs[33]。由此可见,功能内生定殖能够影响植物体内POPs降解代谢的相关酶系活性,进而减少植物体内POPs的污染残留。如何评估功能内生定殖对植物体内POPs代谢酶的表达模式的影响仍需深人研究。
(3) 功能EB降解基因的水平迁移。污染环境对细菌的选择性筛选作用,导致环境中的细菌存在一定程度的基因水平迁移现象,该过程有助于微生物群落快速适应新的环境压力[55]。例如,Wilson等[56]从两种不同地段分离、筛选出相同萘降解功能菌的降解基因,分别属于phnAc和nahAc,证实不同菌株间发生了降解基因的水平迁移。在植物体内功能EB也可能将有益的抗性基因和降解基因水平迁移到其他土著内生菌群中,进而提高整个内生菌群对污染物的降解代谢功能[57]。Taghavi等[55]研究指出,利用含有pTOM-Bu61质粒编码的甲苯降解功能内生伯克氏菌Bu61接种杨树,结果在杨树体内其他内生菌群中也检测到了pTOM-Bu61基因的存在,表明甲苯降解pTOM-Bu61基因由伯克氏菌Bu61水平迁移到其他内生菌群落中,从而增强了整个内生菌群对甲苯的代谢效能。Wang等[58]从玉米植物根部分离、筛选出多株具有苯酚降解特性的功能内生假单胞菌和伯克氏菌,并证实这些功能EB的邻苯二酚-2, 3-双加氧酶质粒基因能够水平迁移到其他内生菌和根际细菌体内,促进整个微生物群落对苯酚的代谢作用。因此,内生群落中的基因水平迁移能够促使植物体内土著微生物获得新的降解特性、提高整个微生物群落对POPs的降解潜能,从而增强植物体内POPs的代谢效能。
(4) 功能内生细菌代谢基因的丰度和表达。研究已经证实,重金属和有机污染物能够促进功能细菌相关抗性和代谢基因在环境中的丰度和表达量[49, 59-61]。例如,Altimira等[62]研究指出,在Cu污染的土壤中存在由copA基因编码的多铜氧化酶,该酶可以促进细菌产生Cu抗性基因并大量表达; 然而,在没有Cu污染的土壤中并未发现该基因存在。由此可见,功能EB定殖在污染植物体内能够增加相关代谢基因的表达丰度,促进植物体内重金属的解毒作用或POPs的代谢潜能。Sicilian等[63]研究了不同污染位点植物体内功能内生菌群中相关代谢基因的表达量,发现在石油烃污染的位点,植物体内可培养功能内生群落中烷烃单加氧酶(alkB)基因型是土壤微生物群落中的10倍;而根际细菌群落提取的总DNA中萘双加氧酶(ndoB)基因普遍高于土壤; 在硝基芳香化合物污染的位点,植物根中内生菌群2-硝基甲苯还原酶(ntdAa)基因普遍高于周围土壤;同样,从根中内生群落提取的硝基甲苯单加氧酶(ntnM)基因是非根际土壤中的14倍。这些结果清晰地表明植物受到有机污染物胁迫时能够促进功能EB相关代谢基因在植物体内的大量表达。因此,将功能EB定殖在POPs污染的植物体内有望促进其相关代谢基因的大量表达,从而增强植物体内POPs的降解代谢作用。
3 结论与展望基于以上概括总结,从POPs污染区的健康植物体内分离、筛选出具有POPs降解特性的EB,并将其重新定殖在目标农作物体内,能够规避作物体内POPs的污染残留风险。该方法具有操作简便、经济有效、环境友好和无二次污染等优点,有望为防控土壤POPs污染、消除作物体内POPs污染风险、保障污染区农产品安全和人群健康等提供一种新型的绿色环保技术,具有较大的开发空间和应用价值[28, 38, 49]。然而,该方法仍需要解决以下几个技术问题:
(1) 农作物吸收、代谢POPs的过程中,可通过区室化作用将POPs及其代谢中间体分配到各个亚细胞组分中。功能EB定殖在宿主作物的维管束导管、细胞间隙或细胞内部,可能改变POPs在作物体内的亚细胞分配行为和代谢途径。因此,尝试从亚细胞水平剖析功能EB定殖在农作物体内调控其POPs的亚细胞分配和代谢产物分布的影响,并阐明其作用机理至关重要。
(2) 定殖方法能够显著地影响POPs降解功能EB在目标农作物体内的定殖分布、数量变化及其对植物—402—体内POPs的降解效能[10, 64]。选择不同的定殖方式,如灌根、浸种、浸根和涂叶等,明确不同定殖方法对功能EB在目标农作物体内的定殖规律及其对农作物体内POPs降解代谢的影响,从而根据实际情况优选出最佳的定殖方法用于规避POPs污染区作物体内有机污染风险。
(3) 采用宏基因组学,将与POPs代谢相关的功能基因转移到土著EB或作物基因组中,构建出高效基因降解工程菌或作物,从而达到有效规避作物体内POPs污染的目的[65-66]。与接种外源功能EB相比,该技术不仅避免了外源功能EB与不同宿主作物之间存在的特异性选择现象,也增强了土著EB或作物自身对POPs的降解效能,有望在大规模的农业污染区域得到广泛应用。
| [1] | Chen Y, Wang C X, Wang Z J. Residues and source identification of persistent organic pollutants in farmland soils irrigated by effluents from biological treatment plants[J]. Environment International, 2005, 31(6): 778–783. DOI:10.1016/j.envint.2005.05.024 |
| [2] | Hurtado C, Domínguez C, Pérez-Babace L, et al. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions[J]. Journal of Hazardous Materials, 2016, 305: 139–148. DOI:10.1016/j.jhazmat.2015.11.039 |
| [3] | Scheringer M, Salzmann M, Stroebe M, et al. Long-range transport and global fractionation of POPs:Insights from multimedia modeling studies[J]. Environmental Pollution, 2004, 128(1): 177–188. |
| [4] | Gao Y, Zhu L. Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils[J]. Chemosphere, 2004, 55(9): 1169–1178. DOI:10.1016/j.chemosphere.2004.01.037 |
| [5] | Chiou C T, Sheng G, Manes M. A partition-limited model for the plant uptake of organic contaminants from soil and water[J]. Environmental Science & Technology, 2001, 35(7): 1437–1444. |
| [6] | Fryer M E, Collins C D. Model intercomparison for the uptake of organic chemicals by plants[J]. Environmental Science & Technology, 2003, 37(8): 1617–1624. |
| [7] | Hung H, Thomas G O, Jones K C, et al. Grass-air exchange of polychlorinated biphenyls[J]. Environmental Science & Technology, 2001, 35(20): 4066–4073. |
| [8] | Kvesitadze E, Sadunishvili T, Kvesitadze G. Mechanisms of organic contaminants uptake and degradation in plants[J]. World Academy of Science, Engineering and Technology, 2009, 55(6): 458–468. |
| [9] | Parrish Z D, White J C, Isleyen M, et al. Accumulation of weathered polycyclic aromatic hydrocarbons(PAHs) by plant and earthworm species[J]. Chemosphere, 2006, 64(4): 609–618. DOI:10.1016/j.chemosphere.2005.11.003 |
| [10] | Afzal M, Khan Q M, Sessitsch A. Endophytic bacteria:Prospects and applications for the phytoremediation of organic pollutants[J]. Chemosphere, 2014, 117: 232–242. DOI:10.1016/j.chemosphere.2014.06.078 |
| [11] | Brader G, Compant S, Mitter B, et al. Metabolic potential of endophytic bacteria[J]. Current Opinion in Biotechnology, 2014, 27: 30–37. DOI:10.1016/j.copbio.2013.09.012 |
| [12] | Samsøe-Petersen L, Larsen E H, Larsen P B, et al. Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils[J]. Environmental Science & Technology, 2002, 36(14): 3057–3063. |
| [13] | Fismes J, Perrin-Ganier C, Empereur-Bissonnet P, et al. Soil-to-root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils[J]. Journal of Environmental Quality, 2002, 31(5): 1649–1656. DOI:10.2134/jeq2002.1649 |
| [14] |
丁海涛, 李顺鹏, 沈标, 等.
拟除虫菊酯类农药残留降解菌的筛选及其生理特性研究[J]. 土壤学报, 2003, 40(1): 123–129.
DING Hai-tao, LI Shun-peng, SHEN Biao, et al. Isolation of pyrethroids degrading strain and its physiological characteristics[J]. Acta Pedologica Sinica, 2003, 40(1): 123–129. DOI:10.11766/trxb200012250117 (in Chinese) |
| [15] |
毕新慧, 储少岗, 徐晓白.
多氯联苯在水稻田中的迁移行为[J]. 环境科学学报, 2001, 21(4): 454–458.
BI Xin-hui, CHU Shao-gang, XU Xiao-bai. Transport of PCBs in contaminated paddy fields[J]. Acta Scientiae Chrcumstantiae, 2001, 21(4): 454–458. (in Chinese) |
| [16] | White J C. Differential bioavailability of field-weathered p, p'-DDT to plants of the Cucurbit and Cucumis genera[J]. Chemosphere, 2002, 49: 143–152. DOI:10.1016/S0045-6535(02)00277-1 |
| [17] | Åslund M L W, Zeeb B A, Rutter A, et al. In situ phytoextraction of polychlorinated biphenyl-(PCB) contaminated soil[J]. Science of the Total Environment, 2007, 374(1): 1–12. DOI:10.1016/j.scitotenv.2006.11.052 |
| [18] | Mattina M J I, Iannucci-Berger W, Dykas L. Chlordane uptake and its translocation in food crops[J]. Journal of Agricultural and Food Chemistry, 2000, 48(5): 1909–1915. DOI:10.1021/jf990566a |
| [19] | Cai Q Y, Mo C H, Wu Q T, et al. Polycyclic aromatic hydrocarbons and phthalic acid esters in the soil-radish(Raphanus sativus) system with sewage and compost application[J]. Bioresourse Technology, 2008, 99(6): 1830–1836. DOI:10.1016/j.biortech.2007.03.035 |
| [20] |
刘征涛.
持久性有机污染物的主要特征和研究进展[J]. 环境科学研究, 2005, 18(3): 93–102.
LIU Zheng-tao. Environmental behavior characteristics and research progress of persistent organic pollutants[J]. Research of Environmental Sciences, 2005, 18(3): 93–102. (in Chinese) |
| [21] | Borga K, Wolkers H, Skaare J U, et al. Bioaccumulation of PCBs in Arctic seabirds:Influence of dietary exposure and congener biotransformation[J]. Environmental Pollution, 2005, 134(3): 397–409. DOI:10.1016/j.envpol.2004.09.016 |
| [22] | Capuano F, Cavalchi B, Martinelli G, et al. Environmental prospection for PCDD/PCDF, PAH, PCB and heavy metals around the incinerator power plant of Reggio Emilia town(Northern Italy) and surrounding main roads[J]. Chemosphere, 2005, 58(11): 1563–1569. DOI:10.1016/j.chemosphere.2004.11.065 |
| [23] | Kang F X, Chen D S, Gao Y Z, et al. Distribution of polycyclic aromatic hydrocarbons in subcellular root tissues of ryegrass(Lolium multiflorum Lam.)[J]. BMC Plant Biology, 2010, 10(1): 210. DOI:10.1186/1471-2229-10-210 |
| [24] | Gao Y Z, Collins C D. Uptake pathways of polycyclic aromatic hydrocarbons in white clover[J]. Environmental Science & Technology, 2009, 43(16): 6190–6195. |
| [25] | Sterling T M, Balke N E. Differential bentazon metabolism and retention of bentazon metabolites by plant cell cultures[J]. Pesticide Biochemistry and Physiology, 1989, 34(1): 39–48. DOI:10.1016/0048-3575(89)90139-9 |
| [26] | Sandermann H. Higher plant metabolism of xenobiotics:The green liver concept[J]. Pharmacogenetics, 1994, 4(5): 225–241. DOI:10.1097/00008571-199410000-00001 |
| [27] | DeRidder B P. Induction of glutathione S-transferases in Arabidopsis by herbicide safeners[J]. Plant Physiology, 2002, 130(3): 1497–1505. DOI:10.1104/pp.010066 |
| [28] | Arslan M, Imran A, Khan Q M, et al. Plant-bacteria partnerships for the remediation of persistent organic pollutants[J]. Environmental Science and Pollution Research, 2015: 1–15. |
| [29] | Reinhold-Hurek B, Hurek T. Living inside plants:Bacterial endophytes[J]. Current Opinion in Plant Biology, 2011, 14(4): 435–443. DOI:10.1016/j.pbi.2011.04.004 |
| [30] | Zhu X, Ni X, Waigi M G, et al. Biodegradation of mixed PAHs by PAH-degrading endophytic bacteria[J]. International Journal of Environmental Research and Public Health, 2016, 13(8): 805. DOI:10.3390/ijerph13080805 |
| [31] | Sessitsch A, Hardoim P, Döring J, et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis[J]. Molecular Plant-Microbe Interactions, 2012, 25(1): 28–36. DOI:10.1094/MPMI-08-11-0204 |
| [32] | Phillips L A, Germida J J, Farrell R E, et al. Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants[J]. Soil Biology and Biochemistry, 2008, 40(12): 3054–3064. DOI:10.1016/j.soilbio.2008.09.006 |
| [33] | Waigi M G, Kang F X, Goikavi C, et al. Phenanthrene biodegradation by sphingomonads and its application in the contaminated soils and sediments:A review[J]. International Biodeterioration & Biodegradation, 2015, 104: 333–349. |
| [34] | Demaneche S, Meyer C, Micoud J, et al. Identification and functional analysis of two aromatic-ring-hydroxylating dioxygenases from a Sphingomonas strain that degrades various polycyclic aromatic hydrocarbons[J]. Applied and Environmental Microbiology, 2004, 70(11): 6714–6725. DOI:10.1128/AEM.70.11.6714-6725.2004 |
| [35] | Khara P, Roy M, Chakraborty J, et al. Functional characterization of diverse ring-hydroxylating oxygenases and induction of complex aromatic catabolic gene clusters in Sphingobium sp. PNB[J]. FEBS Open Bio, 2014(4): 290–300. |
| [36] | Pinyakong O, Habe H, Omori T. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons(PAHs)[J]. The Journal of General and Applied Microbiology, 2003, 49(1): 1–19. DOI:10.2323/jgam.49.1 |
| [37] | Singleton D R, Ramirez L G, Aitken M D. Characterization of a polycyclic aromatic hydrocarbon degradation gene cluster in a phenanthrene-degrading Acidovorax strain[J]. Applied and Environmental Microbiology, 2009, 75(9): 2613–2620. DOI:10.1128/AEM.01955-08 |
| [38] | Sun K, Liu J, Gao Y Z, et al. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp[J]. Scientific Reports, 2014: 4. |
| [39] | Weyens N, Van Der Lelie D, Artois T, et al. Bioaugmentation with engineered endophytic bacteria improves contaminant fate in phytoremediation[J]. Environmental Science & Technology, 2009, 43(24): 9413–9418. |
| [40] | Vergani L, Mapelli F, Zanardini E, et al. Phyto-rhizoremediation of polychlorinated biphenyl contaminated soils:An outlook on plant-microbe beneficial interactions[J]. Science of the Total Environment, 2017, 575: 1395–1406. DOI:10.1016/j.scitotenv.2016.09.218 |
| [41] | Banik A, Mukhopadhaya S K, Sahana A, et al. Fluorescence resonance energy transfer(FRET)-based technique for tracking of endophytic bacteria in rice roots[J]. Biology and Fertility of Soils, 2016, 52(2): 277–282. DOI:10.1007/s00374-015-1064-6 |
| [42] | Sun K, Liu J, Jin L, et al. Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination[J]. Plant and Soil, 2014, 374(1-2): 251–262. DOI:10.1007/s11104-013-1875-x |
| [43] | Germaine K J, Liu X, Cabellos G G, et al. Bacterial endophyte-enhanced phytoremediation of the organochlorine herbicide 2, 4-dichlorophenoxyacetic acid[J]. FEMS Microbiology Ecology, 2006, 57(2): 302–310. DOI:10.1111/fem.2006.57.issue-2 |
| [44] | Van Aken B, Yoon J M, Schnoor J L. Biodegradation of nitro-substituted explosives 2, 4, 6-trinitrotoluene, hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine, and octahydro-1, 3, 5, 7-tetranitro-1, 3, 5-tetrazocine by a phytosymbiotic Methylobacterium sp. associated with poplar tissues(Populus deltoids×nigra DN34)[J]. Applied and Environmental Microbiology, 2004, 70(1): 508–517. DOI:10.1128/AEM.70.1.508-517.2004 |
| [45] | Mannisto M K, Tiirola M A, Puhakka J A. Degradation of 2, 3, 4, 6-tetrachlorophenol at low temperature and low dioxygen concentrations by phylogenetically different groundwater and bioreactor bacteria[J]. Biodegradation, 2001, 12(5): 291–301. DOI:10.1023/A:1014362508447 |
| [46] | Wang Y, Yamazoe A, Suzuki S, et al. Isolation and characterization of dibenzofuran-degrading Comamonas sp. strains isolated from white clover roots[J]. Current Microbiology, 2004, 49(4): 288–294. DOI:10.1007/s00284-004-4348-x |
| [47] | Zhu X, Wang W, Crowley D E, et al. The endophytic bacterium Serratia sp. PW7 degrades pyrene in wheat[J]. Environmental Science and Pollution Research, 2017, 24(7): 6648–6656. DOI:10.1007/s11356-016-8345-y |
| [48] | Eevers N, Hawthorne J R, White J C, et al. Exposure of Cucurbita pepo to DDE-contamination alters the endophytic community:A cultivation dependent vs a cultivation independent approach[J]. Environmental Pollution, 2016, 209: 147–154. DOI:10.1016/j.envpol.2015.11.038 |
| [49] | Ijaz A, Imran A, ulHaq M A, et al. Phytoremediation:Recent advances in plant-endophytic synergistic interactions[J]. Plant and Soil, 2016, 405(1-2): 179–195. DOI:10.1007/s11104-015-2606-2 |
| [50] | Thijs S, Sillen W, Rineau F, et al. Towards an enhanced understanding of plant-microbiome interactions to improve phytoremediation:Engineering the metaorganism[J]. Frontiers in Microbiology, 2016(7): 341. |
| [51] | Musilova L, Ridl J, Polivkova M, et al. Effects of secondary plant metabolites on microbial populations:Changes in community structure and metabolic activity in contaminated environments[J]. International Journal of Molecular Sciences, 2016, 17(8): 1205. DOI:10.3390/ijms17081205 |
| [52] | Kim M H, Hao O J. Cometabolic degradation of chlorophenols by Acinetobacter species[J]. Water Research, 1999, 33(2): 562–574. DOI:10.1016/S0043-1354(98)00228-0 |
| [53] | Muratova A, Dubrovskaya E, Golubev S, et al. The coupling of the plant and microbial catabolisms of phenanthrene in the rhizosphere of Medicago sativa[J]. Journal of Plant Physiology, 2015, 188: 1–8. DOI:10.1016/j.jplph.2015.07.014 |
| [54] |
盛月慧, 刘娟, 高彦征, 等.
黑麦草体内POD和PPO活性及可培养内生细菌种群对不同浓度菲污染的响应[J]. 南京农业大学学报, 2013, 36(6): 51–59.
SHENG Yue-hui, LIU Juan, GAO Yan-zheng, et al. Effects of phenanthrene on the POD and PPO activities and endophytic bacteria population characteristicsin ryegrass(Lolium multiflorum Lam.)[J]. Journal of Nanjing Agricultural University, 2013, 36(6): 51–59. DOI:10.7685/j.issn.1000-2030.2013.06.009 (in Chinese) |
| [55] | Taghavi S, Barac T, Greenberg B, et al. Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene[J]. Applied and Environmental Microbiology, 2005, 71(12): 8500–8505. DOI:10.1128/AEM.71.12.8500-8505.2005 |
| [56] | Wilson M S, Herrick J B, Jeon C O, et al. Horizontal transfer of phnAc dioxygenase genes within one of two phenotypically and genotypically distinctive naphthalene-degrading guilds from adjacent soil environments[J]. Applied and Environmental Microbiology, 2003, 69(4): 2172–2181. DOI:10.1128/AEM.69.4.2172-2181.2003 |
| [57] | Li J H, Wang E T, Chen W F, et al. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China[J]. Soil Biology and Biochemistry, 2008, 40(1): 238–246. DOI:10.1016/j.soilbio.2007.08.014 |
| [58] | Wang Y, Xiao M, Geng X, et al. Horizontal transfer of genetic determinants for degradation of phenol between the bacteria living in plant and its rhizosphere[J]. Applied Microbiology and Biotechnology, 2007, 77(3): 733–739. DOI:10.1007/s00253-007-1187-2 |
| [59] | Andria V, Reichenauer T G, Sessitsch A. Expression of alkane monooxygenase(alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass(Lolium multiflorum, L.) grown in diesel contaminated soil[J]. Environmental Pollution, 2009, 157(12): 3347–3350. DOI:10.1016/j.envpol.2009.08.023 |
| [60] | Hendrickx B, Dejonghe W, Faber F, et al. PCR-DGGE method to assess the diversity of BTEX mono-oxygenase genes at contaminated sites[J]. FEMS Microbiology Ecology, 2006, 55(2): 262–273. DOI:10.1111/fem.2006.55.issue-2 |
| [61] | Yousaf S, Afzal M, Reichenauer T G, et al. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains[J]. Environmental Pollution, 2011, 159(10): 2675–2683. DOI:10.1016/j.envpol.2011.05.031 |
| [62] | Altimira F, Yáñez C, Bravo G, et al. Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of central Chile[J]. BMC Microbiology, 2012, 12(1): 193. DOI:10.1186/1471-2180-12-193 |
| [63] | Siciliano S D, Fortin N, Mihoc A, et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination[J]. Applied and Environmental Microbiology, 2001, 67(6): 2469–2475. DOI:10.1128/AEM.67.6.2469-2475.2001 |
| [64] | Liu J, Xiang Y, Zhang Z, et al. Inoculation of a phenanthrene-degrading endophytic bacterium reduces the phenanthrene level and alters the bacterial community structure in wheat[J]. Applied Microbiology and Biotechnology, 2017: 1–14. |
| [65] | Oliveira V, Gomes N, Almeida A, et al. Hydrocarbon contamination and plant species determine the phylogenetic and functional diversity of endophytic degrading bacteria[J]. Molecular Ecology, 2014, 23(6): 1392–1404. DOI:10.1111/mec.12559 |
| [66] | Ren H, Su Y, Zhang J, et al. Recombinant protein, AlnA, combined with transgenic alfalfa remediates polychlorinated biphenyl-contaminated soils:Efficiency and rhizosphere microbial community response[J]. Biotechnology Letters, 2016, 38(11): 1893–1901. DOI:10.1007/s10529-016-2169-1 |
 2017, Vol. 34
2017, Vol. 34